ABSTRACT
Purpose
To evaluate different dissolution testing methods and subsequently develop a simple to perform but reproducible and discriminating dissolution technique for inhalative powders.
Methods
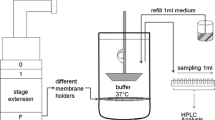
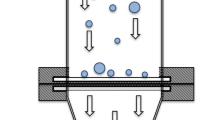
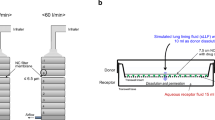
From a dry powder a fraction of aerosolized particles with an aerodynamic particle size below 5 μm was collected on regenerated cellulose membranes using an abbreviated Andersen cascade impactor. The membrane was then transferred to the respective dissolution set up either paddle apparatus with membrane holder, flow through cell or Franz diffusion cell.
Results
All tested dissolution techniques could discriminate between good and poorly soluble substances, but only the paddle apparatus differentiated between small variations of solubility. We showed that membrane coverage and particle diameter play an important role for the dissolution rate. The profiles were fitted with mathematical models (e.g., Weibull, first order) choosing the best fit for determination of the mean dissolution time. Furthermore, a correlation between the dissolution profiles obtained with Franz cell compared to paddle apparatus could be shown.
Conclusion
The paddle apparatus with membrane holder has the best discrimination power with optimal reproducibility.






Similar content being viewed by others
Abbreviations
- ACI:
-
Andersen cascade impactor
- DPI:
-
dry powder inhaler
- HPLC:
-
high performance liquid chromatography
- MDT:
-
mean dissolution time
- PE:
-
polyethylen
- PEEK:
-
polyetherketone
- RC:
-
regenerated cellulose
- SEM:
-
scanning electron microscopy
- SLF:
-
simulated lung fluid
REFERENCES
Davies NM, Feddah MR. A novel method for assessing dissolution of aerosol inhaler products. Int J Pharm. 2003;255(1–2):175–87.
Gray VA, Hickey AJ, Balmer P, Davies NM, Dunbar C, Foster TS, et al. The inhalation ad hoc advisory panel for the USP performance tests of inhalation dosage forms. Pharm Forum. 2008;34(4):1068–74.
Jensen B, Reiners M, Wolkenhauer M, Ritzheim P, May S, Schneider M, et al. Dissolution testing for inhaled products. Respiratory Drug Delivery Europe. 2011. Ref Type: Abstract.
Henning A, Hein S, Schneider M, Bur M, Lehr CM. Pulmonary drug delivery: medicines for inhalation. 197, 171–192. 2010. Ref Type: Serial (Book, Monograph).
Patton JS, Brain JD, Davies LA, Fiegel J, Gumbleton M, Kim KJ, et al. The particle has landed-characterizing the fate of inhaled pharmaceuticals. J Aerosol Med Pulm Drug Deliv. 2010;23 Suppl 2:S71–87.
McConville JT, Patel N, Ditchburn N, Tobyn MJ, Staniforth JN, Woodcock P. Use of a novel modified TSI for the evaluation of controlled-release aerosol formulations. I. Drug Dev Ind Pharm. 2000;26(11):1191–8.
Cook RO, Pannu RK, Kellaway IW. Novel sustained release microspheres for pulmonary drug delivery. J Control Release. 2005;104(1):79–90.
Salama RO, Traini D, Chan HK, Young PM. Preparation and characterisation of controlled release co-spray dried drug-polymer microparticles for inhalation 2: evaluation of in vitro release profiling methodologies for controlled release respiratory aerosols. Eur J Pharm Biopharm. 2008;70(1):145–52.
Son YJ, McConville JT. Development of a standardized dissolution test method for inhaled pharmaceutical formulations. Int J Pharm. 2009;382(1–2):15–22.
Son YJ, Horng M, Copley M, McConville JT. Optimization of an in vitro dissolution test method for inhalation formulations. Dissolut Technol. 2010;17(2):6–13.
Franz TJ. Percutaneous absorption. On the relevance of in vitro data. J Investig Dermatol. 1975;64(3):190–5.
Bur M, Huwer H, Muys L, Lehr CM. Drug transport across pulmonary epithelial cell monolayers: effects of particle size, apical liquid volume, and deposition technique. J Aerosol Med Pulm Drug Deliv. 2010;23(3):119–27.
Jaspart S, Bertholet P, Piel G, Dogné JM, Delattre L, Evrard B. Solid lipid microparticles as a sustained release system for pulmonary drug delivery. Eur J Pharm Biopharm. 2007;65(1):47–56.
United States Pharmacopeial Convention. Dissolution. United States Pharamacopeia and National Formulary. Maryland: 2011.
Petersen H, Kullberg A, Edsbäcker S, Greiff L. Nasal retention of budesonide and fluticasone in man: formation of airway mucosal budesonide-esters in vivo. Br J Clin Pharmacol. 2001;51(2):159–63.
Yoshida F, Topliss JG. QSAR model for drug human oral bioavailability. J Med Chem. 2000;43(13):2575–85.
European Pharmacopoeia 7.3 online. 2012.
Langguth, Fricker, Wunderli-Allenspach. Biopharmazie. Weinheim: Wiley-VCH; 2004.
ACKNOWLEDGMENTS & DISCLOSURES
Wolfgang Bootz (Boehringer Ingelheim) and Dr. Bernhard Meier for SEM pictures
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
DSC of substance A amorphous base: glass transition point is between 6 and 10 minutes at approx. 110 °C (heating rate 10 °C/min) (DOCX 396 kb)
Rights and permissions
About this article
Cite this article
May, S., Jensen, B., Wolkenhauer, M. et al. Dissolution Techniques for In Vitro Testing of Dry Powders for Inhalation. Pharm Res 29, 2157–2166 (2012). https://doi.org/10.1007/s11095-012-0744-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-012-0744-2