Abstract
Purpose
To co-crystallise mannitol and lactose with a view to obtaining crystals with more favourable morphological features than either lactose or mannitol alone, suitable for use as carriers in formulations for dry powder inhalers (DPIs) using simultaneous engineering of lactose-mannitol mixtures.
Methods
Mannitol and lactose individually and the two sugars with three different ratios were crystallised/co-crystallised using anti-solvent precipitation technique. Obtained crystals were sieved to separate 63–90 μm size fractions and then characterised by size, shape, density and in vitro aerosolisation performance. Solid state of crystallized samples was studied using FT-IR, XRPD and DSC.
Results
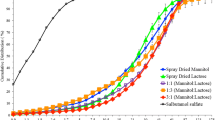
At unequal ratios of mannitol to lactose, the elongated shape dominated in the crystallisation process. However, lactose exerted an opposite effect to that of mannitol by reducing elongation ratio and increasing the crystals’ width and thickness. Crystallised β-lactose showed different anomers compared to commercial lactose (α-lactose monohydrate). Crystallised α-mannitol showed different polymorphic form compared to commercial mannitol (β-mannitol). Crystallised mannitol:lactose showed up to 5 transitions corresponding to α-mannitol, α-lactose monohydrate, β-lactose, 5α-/3β-lactose and 4α-/1β-lactose. In vitro deposition assessments showed that crystallised carriers produced more efficient delivery of salbutamol sulphate compared to formulations containing commercial grade carriers.
Conclusion
The simultaneous crystallization of lactose-mannitol can be used as a new approach to improve the performance of DPI formulations.











Similar content being viewed by others
References
Coates MS, Fletcher DF, Chan HK, Raper JA. Effect of design on the performance of a dry powder inhaler using computational fluid dynamics. Part 1: Grid structure and mouthpiece length. J Pharm Sci. 2004;93:2863–76.
Hindle M, Jashnani RN, Byron PR. Dose emissions from marketed inhalers: Influence of flow, volume and environment. Respiratory Drug Delivery IV. Interpharm Press Inc., Buffalo Grove, IL, USA. 1994;137–42.
Kaialy W, Martin GP, Ticehurst MD, Royall P, Mohammad MA, Murphy J, Nokhodchi A. Characterisation and deposition studies of recrystallised lactose from binary mixtures of ethanol/butanol for improved drug delivery from dry powder inhalers. The AAPS Journal. 2011;13:30–43.
Anderson PJ. Delivery options and devices for aerosolized therapeutics. Chest. 2001;120:89S–93S.
Esmen NA. Adhesion and aerodynamic resuspension of fibrous particles. J Environ Eng. 1996;122:379–84.
Kaialy W, Momin MN, Ticehurst MD, Murphy J, Nokhodchi A. Engineered mannitol as an alternative carrier to enhance deep lung penetration of salbutamol sulphate from dry powder inhaler. Colloid Surface B. 2010;79:345–56.
Jones MD, Harris H, Hooton JC, Shur J, King GS, Mathoulin CA, Nicol K, Smith TL, Dawson ML, Ferrie AR, Price R. An investigation into the relationship between carrier-based dry powder inhalation performance and formulation cohesive-adhesive force balances. Eur J Pharm Biopharm. 2008;69:496–507.
Ooi J, Traini D, Hoe S, Wong W, Young PM. Does carrier size matter? A fundamental study of drug aerosolisation from carrier based dry powder inhalation systems. Int J Pharm. 2011;413:1–9.
Donovan MJ, Smyth HDC. Influence of size and surface roughness of large lactose carrier particles in drypowder inhaler formulations. Int J Pharm. 2010;402:1–9.
Dickhoff BHJ, de Boer AH, Lambregts D, Frijlink HW. The effect of carrier surface and bulk properties on drug particle detachment from crystalline lactose carrier particles during inhalation as a function of carrier payload and mixing time. Eur J PharmBiopharm. 2003;56:291–302.
Shur J, Harris H, Jones MD, Kaerger JS, Price R. The role of fines in the modification of the fluidization and dispersion mechanism within dry powder inhaler formulations. Pharm Res. 2008;25:1931–40.
Donovan MJ, Kim SH, Raman V, Smyth HDC. Dry powder inhaler device influence on carrier particle performance. J Pharm Sci. 2012;101:1097–107.
Guenette E, Barrett A, Kraus D, Brody R, Harding L, Magee G. Understanding the effect of lactose particle size on the properties of DPI formulations using experimental design. Int J Pharm. 2009;280:80–8.
Kaialy W, Martin GP, Ticehurst MD, Momin MN, Nokhodchi A. The enhanced aerosol performance of salbutamol from dry powders containing engineered mannitol as excipient. Int J Pharm. 2010;392:178–88.
Kaialy W, Alhalaweh A, Velaga SP, Nokhodchi A. Effect of carrier particle shape dry powder inhaler performance. Int J Pharm. 2011;421:12–23.
Kaialy W, Martin GP, Larhrib H, Ticehurst MD, Kolosionek E, Nokhodchi A. The influence of physical properties and morphology of crystallised lactose on delivery of salbutamol sulphate from dry powder inhalers. Colloid Surface B. 2011;89:29–39.
Lucas P, Clarke MJ, Anderson K, Tobyn MJ, Staniforth JN. The role of fine partilce excipients in pharmaceutical dry powder aerosols. Drug Delivery to the Lungs VI. 1998;VI:243–50.
Shah KR, Hussain MA, Hubert M, Farag Badawy SI. Form conversion of anhydrous lactose during wet granulation and its effect on compactibility. Int J Pharm. 2008;357:228–34.
Dalby RN, Byron PR, Peart J, Suman JD, Farr SJ. Respiratory drug delivery IX. Palm Desert, California: Davis Healthcare International; 2004.
Nakate T, Yoshida H, Ohike A, Tokunaga Y, Ibuki R, Kawashima Y. Formulation development of inhalation powders for FK888 with carrier lactose using spinhaler® and its absorption in healthy volunteers. J Controlled Release. 2004;97:19–29.
Arnold K, Grass P, Knecht A, Roos R, Sluke G, Thieme H, Wenzel J. Powders for inhalation. US patent. 1995;5478578.
Kawashima Y, Serigano T, Hino T, Yamamoto H, Takeuchi H. Effect of surface morphology of carrier lactose on dry powder inhalation property of pranlukast hydrate. Int J Pharm. 1998;172:179–88.
Rabbani NR, Seville PC. The influence of formulation components on the aerosolisation properties of spray-dried powders. J Controlled Release. 2005;110:130–40.
Zeng XM, Martin GP, Marriott C, Pritchard J. The use of lactose recrystallised from carbopol gels as a carrier for aerosolised salbutamol sulphate. Eur J Pharm Biopharm. 2001;51:55–62.
Traini D, Young PM, Thielmann F, Acharya M. The influence of lactose pseudopolymorphic form on salbutamol Sulfate–Lactose interactions in DPI formulations. Drug Dev Ind Pharm. 2008;34:992–1001.
Young PM, Chan H, Chiou H, Edge S, Tee THS, Traini D. The influence of mechanical processing of dry powder inhaler carriers on drug aerosolization performance. J Pharm Sci. 2007;96:1331–41.
Kaialy W, Ticehurst MD, Murphy J, Nokhodchi A. Improved aerosolization performance of salbutamol sulfate formulated with lactose crystallized from binary mixtures of ethanol—acetone. J Pharm Sci. 2011;100:2665–84.
ASTM D 2488. Standard practice for description and identification of soils (visual–manual procedure). ASTM west conshohocken. 2000.
Yoshinari T, Forbes RT, York P, Kawashima Y. Moisture induced polymorphic transition of mannitol and itsmorphological transformation. Int J Pharm. 2002;247:69–77.
Shamil S, Birch GG, Njoroge S. Intrinsic viscosities and other solution properties of sugars and their possible relation to sweetness. Chem Senses. 1988;13:457.
Shah SP, Misra A. Liposomal amikacin dry powder inhaler: Effect of fines on in vitro performance. AAPS Pharm Sci Tech. 2004;5:107–13.
Kirk JH, Dann SE, Blatchford CG. Lactose: a definitive guide to polymorph determination. Int J Pharm. 2007;334:103–14.
Zimon AD. Adhesion of dust and powder. New York: Plenum; 1982.
Nokhodchi A, Kaialy W, Ticehurst MD. The influence of particle physicochemical properties on delivery of drugs by dry powder inhalers to the lung. In: Popescu MA, editor. Drug delivery book. NY: Hauppauge: Nova; 2011. p. 1–50.
Burger A, Henck JO, Hetz S, Rollinger JM, Weissnicht AA, Stöttner H. Energy/temperature diagram and compression behavior of the polymorphs of D-mannitol. J Pharm Sci. 2000;89:457–68.
Haque M, Roos Y. Crystallization and X-ray diffraction of spray-dried and freeze-dried amorphous lactose. Carbohydr Res. 2005;340:293–301.
Jouppila K, Kansikas J, Roos YH. Crystallization and X–ray diffraction of crystals formed in Water–Plasticized amorphous lactose. Biotechnol Prog. 1998;14:347–50.
Simpson TD, Parrish FW, Nelson ML. Crystalline forms of lactose produced in acidic alcoholic media. J Food Sci. 1982;47:1948–51.
Drapier-Beche N, Fanni J, Parmentier M. Physical and chemical properties of molecular compounds of lactose. J Dairy Sci. 1999;82:2558–63.
Olano A, Bernhard RA, Nickerson TA. Alteration in the ratio of α–to β–lactose co–crystallized from organic solvents. J Food Sci. 1977;42:1066–8.
Kaialy W, Ticehurst MD, Nokhodchi A. Dry powder inhalers: Mechanistic evaluation of lactose formulations containingsalbutamol sulphate. Int J Pharm. 2012;423:184–94.
Tang HK, Chan JA. Prediction of aerodynamic diameter of particles with rough surfaces. Powder Technol. 2004;146:64–78.
Kaialy W, Larhrib H, Nokhodchi A. The effect of carrier particle size on adhesion, content uniformity, and inhalation performance of budesonide using dry powder inhalers. In: Yu-Chaun W, Wei G, editors. Particulate materials: synthesis, characterisation, processing and modelling. Cambridge: Thomas Graham House; 2011. p. 133–9.
Acknowledgments & DISCLOSURES
Waseem Kaialy thanks Dr Ian Slipper (School of Science, University of Greenwich) for taking SEM images. The authors also thank Roquette for providing mannitol samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaialy, W., Larhrib, H., Martin, G.P. et al. The Effect of Engineered Mannitol-Lactose Mixture on Dry Powder Inhaler Performance. Pharm Res 29, 2139–2156 (2012). https://doi.org/10.1007/s11095-012-0743-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-012-0743-3