ABSTRACT
Purpose
To investigate the effects of alternating magnetic fields (AMF) on the death rate of dendritic cells (DCs) loaded with magnetic nanoparticles (MNPs) as heating agents. AMF exposure time and amplitude as well as the MNPs concentration were screened to assess the best conditions for a controlled field-induced cell death.
Methods
Human-monocyte-derived DCs were co-incubated with dextran-coated MNPs. The cells were exposed to AMF (f = 260 kHz; 0 < H0 < 12.7 kA/m) for intervals from 5 to 15 min. Morphology changes were assessed by scanning electron microscopy. Cell viability was measured by Trypan blue and fluorescence-activated cell sorting (FACS) using Annexin-propidium iodide markers.
Results
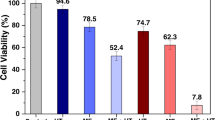
We were able to control the DCs viability by a proper choice AMF amplitude and exposure time, depending on the amount of MNPs uploaded. About 20% of cells showed Annexin-negative/PI-positive staining after 5–10 min of AMF exposure.
Conclusions
Controlled cell death of MNP-loaded DCs can be obtained by adequate tuning of the physical AMF parameters and MNPs concentration. Necrotic-like populations were observed after exposure times as short as 10 min, suggesting a fast underlying mechanism for cell death. Power absorption by the MNPs might locally disrupt endosomic membranes, thus provoking irreversible cell damage.









Similar content being viewed by others
REFERENCES
Woodhall B, Pickrell KL, Georgiade NG, Mahaley MS, Dukes HT. Effect of hyperthermia upon cancer chemotherapy - Application to external cancers of head and face structures. Ann Surg. 1960;151:750–9.
Turnbull AR. Hyperthermia and cancer. Lancet. 1975;1:643–4.
de Bree E, Theodoropoulos PA, Rosing H, Michalakis J, Romanos J, Beijnen JH, et al. Treatment of ovarian cancer using intraperitoneal chemotherapy with taxanes: From laboratory bench to bedside. Cancer Treat Rev. 2006;32:471–82.
Storm FK, Elliott RS, Harrison WH, Morton DL. Clinical rf hyperthermia by magnetic-loop induction - A new approach to human cancer-therapy. IEEE Trans Microw Theory Tech. 1982;30:1149–57.
Jordan A, Wust P, Fahling H, John W, Hinz A, Felix R. Inductive heating of ferrimagnetic particles and magnetic fluids - Physical evaluation of their potential for Hyperthermia. Int J Hyperthermia. 1993;9:51–68.
Magforce. “Magforce nanotechnologies ag receives european regulatory approval for its Nanocancer® therapy.” 2010.
Matsuda H, Tsutsui S, Morita M, Baba K, Kitamura K, Kuwano H, et al. Hyperthermo-chemo-radiotherapy as a definitive treatment for patients with early esophageal-carcinoma. Am J Clin Oncol-Cancer Clin Trials. 1992;15:509–14.
Nie SM. Understanding and overcoming major barriers in cancer nanomedicine. Nanomedicine. 2010;5:523–8.
Marcos-Campos I, Asin L, Torres TE, Marquina C, Tres A, Ibarra MR, et al. Cell death induced by the application of alternating magnetic fields to nanoparticle-loaded dendritic cells. Nanotechnology. 2011;22:13.
Banchereauand J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52.
Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–67.
Trombettaand ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028.
Bodey B, Siegel SE, Kaiser HE. Antigen presentation by dendritic cells and their significance in antineoplastic immunotherapy. In Vivo. 2004;18:81–100.
Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298:1025–9.
Andersson LIM, Hellman P, Eriksson H. Receptor-mediated endocytosis of particles by peripheral dendritic cells. Hum Immunol. 2008;69:625–33.
Molavi L, Mahmud A, Hamdy S, Hung RW, Lai R, Samuel J, et al. Development of a Poly(D, L-lactic-co-glycolic acid) Nanoparticle Formulation of STAT3 Inhibitor JSI-124: Implication for Cancer Immunotherapy. Mol Pharm. 2010;7:364–74.
Basic specifications from the provider includes: Hydrodynamic size: 250 nm; NP concentration:10 mg/ml (4.9x1011 MNPs/ml); Polydispersity index < 0.2; MS (H = 1 T) = 67 emu/g., 2009.
Martin-Saavedra FM, Ruiz-Hernandez E, Bore A, Arcos D, Vallet-Regi M, Vilaboa N. Magnetic mesoporous silica spheres for hyperthermia therapy. Acta Biomaterialia. 2010;6:4522–4531.
Fortin JP, Gazeau F, Wilhelm C. Intracellular heating of living cells through Neel relaxation of magnetic nanoparticles. Eur Biophys J Biophys Lett. 2008;37:223–8.
Rodriguez-Luccioni HL, Latorre-Esteves M, Mendez-Vega J, Soto O, Rodriguez AR, Rinaldi C, et al. Enhanced reduction in cell viability by hyperthermia induced by magnetic nanoparticles. Int J Nanomed. 2011;6:373–380.
Duguet E, Hardel L, Vasseur S. Cell targeting and magnetically induced hyperthermia. Thermal nanosystems and nanomaterials, vol. 118. Berlin: Springer-Verlag Berlin; 2009. p. 343–65.
Prasad NK, Rathinasamy K, Panda D, Bahadur D. Mechanism of cell death induced by magnetic hyperthermia with nanoparticles of gamma-MnxFe2-xO3 synthesized by a single step process. J Mater Chem. 2007;17:5042–51.
Rabin Y. Is intracellular hyperthermia superior to extracellular hyperthermia in the thermal sense? Int J Hyperthermia. 2002;18:194–202.
Villanueva A, de la Presa P, Alonso JM, Rueda T, Martinez A, Crespo P, et al. Hyperthermia HeLa Cell Treatment with Silica-Coated Manganese Oxide Nanoparticles. J Phys Chem C. 2010;114:1976–81.
Creixell M, Bohorquez AC, Torres-Lugo M, Rinaldi C. EGFR-targeted magnetic nanoparticle heaters kill cancer cells without a perceptible temperature rise. ACS Nano. 2011;5:7124–7129.
Lunov O, Zablotskii V, Pastor JM, Perez-Landazabal JI, Gomez-Polo C, Syrovets T, et al. Thermal Destruction on the Nanoscale: Cell Membrane Hyperthermia with Functionalized Magnetic Nanoparticles. In: Hafeli U, Schutt W, Zborowski M, editors. 8th Internatioanl Conference on the Scientific and Clinical Applications of Magnetic Carriers, Vol. 1311, Amer Inst Physics, Melville, 2010, pp. 288–292.
Beaune G, Levy M, Neveu S, Gazeau F, Wilhelm C, Menager C. Different localizations of hydrophobic magnetic nanoparticles within vesicles trigger their efficiency as magnetic nano-heaters. Soft Matter. 2011;7:6248–54.
Acknowledgments & Disclosures
This work was supported by the Spanish Ministerio de Ciencia e Innovación (project MICINN MAT2010-19326 and CONSOLIDER NANOBIOMED CS-27 2006) and IBERCAJA. LA acknowledges MICINN by financial support through a FPU fellowship. The University of Zaragoza, along with their researchers, have filled patents related to the technology and intellectual property reported here. G.F.G. and M.R. I. have equity in nB Nanoscale Biomagnetics S.L. The other authors declare that they do not have any affiliations that would lead to conflict of interest. We are grateful to the I + CS staff (University Hospital, Zaragoza) Dr. J. Godino (FACS experiments) and Dr. M. Royo Cañas (confocal microscopy) for their advice and technical support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1215 kb)
Rights and permissions
About this article
Cite this article
Asín, L., Ibarra, M.R., Tres, A. et al. Controlled Cell Death by Magnetic Hyperthermia: Effects of Exposure Time, Field Amplitude, and Nanoparticle Concentration. Pharm Res 29, 1319–1327 (2012). https://doi.org/10.1007/s11095-012-0710-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-012-0710-z