ABSTRACT
Purpose
Polyelectrolyte complex nanoparticles are a promising vehicle for siRNA delivery but suffer from low stability under physiological conditions. An effective stabilization method is essential for the success of polycationic nanoparticle-mediated siRNA delivery. In this study, sodium triphosphate (TPP), an ionic crosslinking agent, is used to stabilize siRNA-containing nanoparticles by co-condensation.
Methods
siRNA and TPP were co-encapsulated into a block copolymer, poly(ethylene glycol)-b-polyphosphoramidate (PEG-b-PPA), to form ternary nanoparticles. Physicochemical characterization was performed by dynamic light scattering and gel electrophoresis. Gene silencing efficiency in cell lines was assessed by dual luciferase assay system.
Results
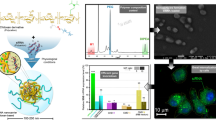
The PEG-b-PPA/siRNA/TPP ternary nanoparticles exhibited high uniformity with smaller size (80–100 nm) compared with PEG-b-PPA/siRNA nanoparticles and showed increased stability in physiological ionic strength and serum-containing medium, due to the stabilization effect from ionic crosslinks between negatively charged TPP and cationic PPA segment. Transfection and gene silencing efficiency of the TPP-crosslinked nanoparticles were markedly improved over PEG-b-PPA/siRNA complexes in serum-containing medium. No significant difference in cell viability was observed between nanoparticles prepared with and without TPP co-condensation.
Conclusions
These results demonstrated the effectiveness of TPP co-condensation in compacting polycation/siRNA nanoparticles, improving nanoparticle stability and enhancing the transfection and knockdown efficiency in serum-containing medium.






Similar content being viewed by others
REFERENCES
Kim DH, Behlke MA, Rose SD, Chang MS, Choi S, Rossi JJ. Synthetic dsRNA Dicer substrates enhance RNAi potency and efficacy. Nat Biotechnol. 2005;23:222–6.
Elbashir SM, Lendeckel W, Tuschl T. RNA interference is mediated by 21-and 22-nucleotide RNAs. Genes Dev. 2001;15:188–200.
Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–8.
Grimmand D, Kay MA. Therapeutic application of RNAi: is mRNA targeting finally ready for prime time? J Clin Investig. 2007;117:3633–41.
Manoharan M. RNA interference and chemically modified small interfering RNAs. Curr Opin Chem Biol. 2004;8:570–9.
Guo PX, Coban O, Snead NM, Trebley J, Hoeprich S, Guo SC, et al. Engineering RNA for targeted siRNA delivery and medical application. Adv Drug Deliv Rev. 2010;62:650–66.
Nothisen M, Kotera M, Voirin E, Remy JS, Behr JP. Cationic siRNAs provide carrier-free gene silencing in animal cells. J Am Chem Soc. 2009;131:17730–1.
Eguchiand A, Dowdy SF. siRNA delivery using peptide transduction domains. Trends Pharmacol Sci. 2009;30:341–5.
Frohlichand T, Wagner E. Peptide- and polymer-based delivery of therapeutic RNA. Soft Matter. 2010;6:226–34.
David S, Pitard B, Benoit JP, Passirani C. Non-viral nanosystems for systemic siRNA delivery. Pharmacol Res. 2010;62:100–14.
Jeong JH, Kim SW, Park TG. Molecular design of functional polymers for gene therapy. Prog Polym Sci. 2007;32:1239–74.
Khurana B, Goyal AK, Budhiraja A, Arora D, Vyas SP. siRNA delivery using nanocarriers—an efficient tool for gene silencing. Curr Gene Ther. 2010;10:139–55.
Mok H, Lee SH, Park JW, Park TG. Multimeric small interfering ribonucleic acid for highly efficient sequence-specific gene silencing. Nat Mater. 2010;9:272–8.
Bolcato-Bellemin AL, Bonnet ME, Creusatt G, Erbacher P, Behr JP. Sticky overhangs enhance siRNA-mediated gene silencing. Proc Natl Acad Sci USA. 2007;104:16050–5.
Raemdonck K, Van Thienen TG, Vandenbroucke RE, Sanders NN, Demeester J, De Smedt SC. Dextran microgels for time-controlled delivery of siRNA. Adv Funct Mater. 2008;18:993–1001.
Tamura A, Oishi M, Nagasaki Y. Enhanced cytoplasmic delivery of siRNA using a stabilized polyion complex based on PEGylated nanogels with a cross-linked polyamine structure. Biomacromolecules. 2009;10:1818–27.
Li J, Chen YC, Tseng YC, Mozumdar S, Huang L. Biodegradable calcium phosphate nanoparticle with lipid coating for systemic siRNA delivery. J Control Release. 2010;142:416–21.
Matsumoto S, Christie RJ, Nishiyama N, Miyata K, Ishii A, Oba M, et al. Environment-responsive block copolymer micelles with a disulfide cross-linked core for enhanced siRNA delivery. Biomacromolecules. 2009;10:119–27.
Jiang X, Dai H, Ke CY, Mo X, Torbenson MS, Li ZP, et al. PEG-b-PPA/DNA micelles improve transgene expression in rat liver through intrabiliary infusion. J Control Release. 2007;122:297–304.
Jiang X, Zheng Y, Chen HH, Leong KW, Wang TH, Mao HQ. Dual-sensitive micellar nanoparticles regulate DNA unpacking and enhance gene-delivery efficiency. Adv Mater. 22:2556–60.
Allen TM, Hansen C, Martin F, Redemann C, Yauyoung A. Liposomes containing synthetic lipid derivatives of poly(ethylene glycol) show prolonged circulation half-lives invivo. Biochim Biophys Acta. 1991;1066:29–36.
Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268:235–7.
Calvo P, RemunanLopez C, VilaJato JL, Alonso MJ. Chitosan and chitosan ethylene oxide propylene oxide block copolymer nanoparticles as novel carriers for proteins and vaccines. Pharm Res. 1997;14:1431–6.
Calvo P, RemunanLopez C, VilaJato JL, Alonso MJ. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J Appl Polym Sci. 1997;63:125–32.
Park JH, Saravanakumar G, Kim K, Kwon IC. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv Drug Deliv Rev. 2010;62:28–41.
Mao SR, Sun W, Kissel T. Chitosan-based formulations for delivery of DNA and siRNA. Adv Drug Deliv Rev. 2010;62:12–27.
Nasti A, Zaki NM, de Leonardis P, Ungphaiboon S, Sansongsak P, Rimoli MG, et al. Chitosan/TPP and chitosan/TPP-hyaluronic acid nanoparticles: systematic optimisation of the preparative process and preliminary biological evaluation. Pharm Res. 2009;26:1918–30.
Bakeev KN, Izumrudov VA, Kuchanov SI, Zezin AB, Kabanov VA. Kinetics and mechanism of interpolyelectrolyte exchange and addition-reactions. Macromolecules. 1992;25:4249–54.
Katayoseand S, Kataoka K. Water-soluble polyion complex associates of DNA and poly(ethylene glycol)-poly(L-lysine) block copolymer. Bioconjug Chem. 1997;8:702–7.
Ishida O, Maruyama K, Tanahashi H, Iwatsuru M, Sasaki K, Eriguchi M, et al. Liposomes bearing polyethyleneglycol-coupled transferrin with intracellular targeting property to the solid tumors in vivo. Pharm Res. 2001;18:1042–8.
Litzinger DC, Buiting AMJ, Vanrooijen N, Huang L. Effect of liposome size on the circulation time and intraorgan distribution of amphipathic poly(ethylene glycol)-containing liposomes. Biochim Biophys Acta, Biomembr. 1994;1190:99–107.
Uchiyama K, Nagayasu A, Yamagiwa Y, Nishida T, Harashima H, Kiwada H. Effects of the size and fluidity of liposomes on their accumulation in tumors—a presumption of their interaction with tumors. Int J Pharm. 1995;121:195–203.
Akitaand H, Harashima H. Advances in non-viral gene delivery: using multifunctional envelope-type nano-device. Expert Opin Drug Deliv. 2008;5:847–59.
ACKNOWLEDGMENTS
The authors thank Dr. James F. Dillman III and Dr. Albert L. Ruff at the Cell and Molecular Biology Branch, US Army Medical Research Institute of Chemical Defense, and Dr. Xuan Jiang at Department of Materials Science and Engineering, Johns Hopkins University, for discussions throughout the study. We thank Dr. Noriko Esumi for providing D407 cells. This study was supported by US Army Defense Threat Reduction Agency through the Grant W81XWH-10-2-0053.
Author information
Authors and Affiliations
Corresponding author
Additional information
Masataka Nakanishi and Rajesh Patil contributed equally to this work.
Rights and permissions
About this article
Cite this article
Nakanishi, M., Patil, R., Ren, Y. et al. Enhanced Stability and Knockdown Efficiency of Poly(ethylene glycol)-b-polyphosphoramidate/siRNA Micellar Nanoparticles by Co-condensation with Sodium Triphosphate. Pharm Res 28, 1723–1732 (2011). https://doi.org/10.1007/s11095-011-0408-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-011-0408-7