ABSTRACT
Purpose
At laboratory scale, the most widely applied methods in therapeutic microencapsulation are based on emulsification using organic solvents. Here, glycofurol was proposed as non-toxic solvent to circumvent these inconveniences using a quasi-emulsion extraction method for the preparation of poly (lactide-co-glycolide) microspheres.
Methods
Matrix polymer and lipophilic drug were dissolved in glycofurol, building the internal phase, and emulsified under stirring into various external phases before microspheres could be obtained and characterized for their pharmacotechnical properties.
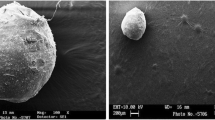
Results
Microspheres were spherical with particle diameters around 100 to 200 μm and also showed a monomodal particle size distribution. The internal sponge-like structure was related to an incomplete glycofurol extraction (residual content: 16.9% ± 1.6% of total particle mass), which is, however, no toxicological drawback. The encapsulation rate of several model compounds increased with rising partition coefficient (Ibuprofen: 1.9% ± 0.6%, Ritonavir: 11.2% ± 0.4%, Lopinavir: 14.0% ± 2.2%, Sudan III: 28.3% ± 0.4%) due to the decreasing solubility in the external phase. In-vitro release kinetics were varying from a complete release after 4 h for Ritonavir to 3 weeks for Sudan III.
Conclusion
This new method was confirmed to be suitable for the preparation of microspheres with the use of a non-toxic solvent and to allow for the entrapment of lipophilic actives and their controlled release.






Similar content being viewed by others
REFERENCES
Wischke C, Schwendeman SP. Principles of encapsulating hydrophobic drugs in PLA/PLGA Microparticles. International Journal of Pharmaceutics. 2008;364:298–327.
Jain RA. The manufacturing techniques of various drug loaded biodegradable poly (lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21:2475–90.
Freitas S, Merkle HP, Gander B. Microencapsulation by solvent extraction/evaporation: reviewing the state of the art of microsphere preparation process technology. Journal of Controlled Release. 2005;102:313–32.
McGinity JW, O’Donnell PB. Preparation of microspheres by the solvent evaporation technique. Advanced Drug Delivery Review. 1997;28:25–42.
Matsumoto A et al. A novel preparation method for PLGA microspheres using non-halogenated solvents. Journal of Controlled Release. 2008;129:223–7.
Lamprecht A et al. Microsphere design for the colonic delivery of 5-fluorouracil. Journal of Controlled Release. 2003;90:313–22.
Residual solvents/ Organic volatile impurities, United States Pharmacopeia 32-NF: chapter 467.
Lösungsmittel-Rückstände, European Pharmacopoeia 6.0/5.04.00.00:869-879.
Bitz C, Doelker E. Influence of the preparation method on residual solvents in biodegradable microspheres. International Journal of Pharmaceutics. 1996;131:171–81.
Witschi C, Doelker E. Residual solvents in pharmaceutical products: acceptable limits, influences on physicochemical properties, analytical methods and documented values. European Journal of Pharmaceutics and Biopharmaceutics. 1996;43:215–42.
Klose D, Siepmann F, et al. PLGA-based drug delivery systems: importance of the type of drug and device geometry. International Journal of Pharmaceutics. 2008;354:95–103.
Sah H. Microencapsulation techniques using ethyl acetate as a dispersed solvent: effects of its extraction rate on the characteristics of PLGA microspheres. Journal of Controlled Release. 1997;129:223–7.
Sah H. Ethyl formate—alternative dispersed solvent useful in preparing PLGA microspheres. International Journal of Pharmaceutics. 2000;195:103–13.
Sah H, Smith MS, Chern RT. A novel method of preparing PLGA microcapsules utilizing methylethyl ketone. Pharmaceutical Research. 1996;13:360–7.
Kranz H, Bodmeier R. A novel in situ forming drug delivery system for controlled parenteral drug delivery. International Journal of Pharmaceutics. 2006;332:107–14.
Aubert-Pouёssel A, Venier-Julienne MC, Saulnier P, Sergent M, Benoit JP. Preparation of PLGA microspheres by an emulsion-extraction process using glycofurol as polymer solvent. Pharmaceutical Research. 2004;21:2384–91.
P.J. Weller. Glycofurol. In R.C. Rowe, P.J. Sheskey, and S. C. Owen. Handbook of Pharmaceutical excipients (sixth edition), PhP, London/ Chicago, 2009, pp. 297-299.
Crowther MA, Pilling A, Owen K. The evaluation of glycofurol as a vehicle for use in toxicity studies. Human Experimental Toxicology. 1997;16:406.
Jeffery H, Davis SS, O’Hagan DT. The preparation and characterization of poly (lactide-co-glycolide) microparticles. II. The entrapment of a model protein using a (water-in-oil)-in-water emulsion solvent evaporation technique. Pharmaceutical Research. 1993;10:362–8.
J.-H. Lee, T.G. Park and Hoo-Kyun Choi. Effect of formulation and processing variables on the characteristics of microspheres for water-soluble drugs prepared by W/O/O double emulsion solvent diffusion method, International Journal of Pharmaceutics 196:75-83 (2000).
H. Zhao, J. Gagnon and Urs O Häfeli. Process and formulation variables in the preparation of injectable and biodegradable magnetic microspheres, Biomagnetic Research and Technology 5:2 (2007).
Conti B, Genta I, Modena T, Pavanetto F. Investigation on process parameters involved in polylactide-co-glycolide microsphere preparation. Drug Development and Industrial Pharmacy. 1995;21:615–22.
Khossravi M et al. Analysis methods of polysorbate 20: A new method to assess the stability of polysorbate 20 and established methods that may overlook degraded polysorbate 20. Pharmaceutical Research. 2002;19:634–9.
Lamprecht A. J-L. Saumet and J-P. Benoit. Lipid nanocarriers as drug delivery system for ibuprofen in pain treatment. International Journal of Pharmaceutics. 2004;278:407–14.
M. Nagase, T. Matsueda and Yasuhiko Osaki. Determination of Sudan III, Sudan IV and Sudan Red 7B in water by high performance liquid chromatography after mixing extraction, Analytical Sciences 5:157-160(1989).
Takahashi M et al. Conventional HPLC method used for simultaneous determination of the seven HIV protease inhibitors and nonnucleoside reverse transcription inhibitor efavirenz in human plasma. Biological & Pharmaceutical Bulletin. 2005;28:1286–90.
Beetge E et al. The influence of the physicochemical characteristics and pharmacokinetic properties of selected NSAID’s on their transdermal absorption. International Journal of Pharmaceutics. 2000;193:261–4.
Kasim NA et al. Molecular properties of WHO essential drugs and provisional biopharmaceutical classification. Molecular Pharmaceutics. 2004;1:85–96.
http://www.drugbank.ca (accessed 04/10/10).
Garg R, Patel D. Hydrophobicity in the design of P2/P2’ tetrahydropyrimidinone HIV protease inhibitors. Bioorganic & Medicinal Chemistry Letters. 2005;15:3767–70.
Bhhatarai B, Garg R. From SAR to comparative QSAR: role of hydrophobicity in the design of 4-hydroxy-5, 6-dihydropyran-2-ones HIV-1 protease inhibitors. Bioorganic & Medicinal chemistry. 2005;13:4078–84.
Williams GC, Sinko PJ. Oral absorption of the HIV protease inhibitors: a current update. Advanced Drug Delivery Reviews. 1999;39:211–38.
Juarranz A, Horobin RW, Proctor GB. Prediction of in situ fluorescence of histochemical reagents using a structure-staining correlation procedure. Histochemistry. 1986;84:426–31.
Abraham MH, Mital A, Zissimos AM. The lipophilicity of Sudan I and its tautomeric forms. Physical Chemistry Chemical Physics. 2002;4:5748–52.
Murakami H et al. Preparation of poly (DL-lactide-co-glycolide) nanoparticles by modified spontaneous emulsification solvent diffusion method. International Journal of Pharmaceutics. 1999;187:143–52.
Wang S, Guo S. Formation mechanism and release behaviour of poly (epsilon-caprolactone) microspheres containing disodium norcantharidate. European Journal of Pharmaceutics and Biopharmaceutics. 2008;69:1176–81.
Niwa T et al. In vitro drug release behavior of D, L-lactide/glycolide copolymer (PLGA) nanospheres with nafarelin acetate prepared by a novel spontaneous emulsification solvent diffusion method. Journal of Pharmaceutical Sciences. 1993;83:727–32.
Lamprecht A et al. Design of pH-sensitive microspheres for the colon delivery of the immune suppressive drug tacrolimus. European Journal of Pharmaceutics and Biopharmaceutics. 2004;58:37–43.
Godbee J et al. Role of solvent/non-solvent ratio on microsphere formation using the solvent removal method. Journal of Microencapsulation. 2004;21:151–60.
ACKNOWLEDGEMENTS
Alf Lamprecht is grateful to the “Institut Universitaire de France” for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Allhenn, D., Lamprecht, A. Microsphere Preparation Using the Untoxic Solvent Glycofurol. Pharm Res 28, 563–571 (2011). https://doi.org/10.1007/s11095-010-0304-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-010-0304-6