ABSTRACT
Purpose
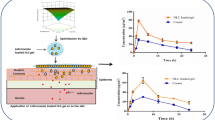
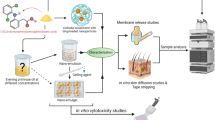
This work probed the topical delivery and skin-staining properties of a novel co-drug, naproxyl-dithranol (Nap-DTH), which comprises anti-inflammatory (naproxen) and anti-proliferative (dithranol) moieties.
Method
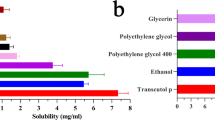
Freshly excised, full-thickness porcine ear skin was dosed with saturated solutions of the compounds. After 24 h, the skin was recovered and used to prepare comparative depth profiles by the tape-stripping technique and to examine the extent of skin staining.
Results
Depth profiles showed that Nap-DTH led to a 5-fold increase in drug retention in the skin compared to dithranol. The application of Nap-DTH also demonstrated improved stability, resulting in lower levels of dithranol degradation products in the skin. Furthermore, significantly less naproxen from hydrolysed Nap-DTH permeated into the receptor phase compared to naproxen when applied alone (0.08 ± 0.03 nmol cm-² and 180 ± 60 nmol cm-², respectively). Moreover, the reduced staining of the skin was very apparent for Nap-DTH compared to dithranol.
Conclusions
Topical delivery of Nap-DTH not only improves the delivery of naproxen and dithranol, but also reduces unwanted effects of the parent moieties, in particular the skin staining, which is a major issue concerning the use of dithranol.






Similar content being viewed by others
Abbreviations
- HMPA:
-
Hexamethylphosphoramide
- IPM:
-
Isopropyl myristate
- LOD:
-
limit of detection
- MW:
-
molecular weight
- Nap-DTH:
-
Naproxyl-dithranol
- SC:
-
stratum corneum
- THF:
-
Tetrahydrofuran
REFERENCES
National Psoriasis Foundation. About psoriasis: statistics. http://www.psoriasis.org/about/stats/ (accessed 19 May 2008).
Jullien D. Psoriasis physiopathology. J Eur Acad Dermatol Venereol. 2006;20:10–23.
McKay IA, Leigh IM. Altered keratinocyte growth and differentiation in psoriasis. Clin Dermatol. 1995;13:105–14.
Tschachler E. Psoriasis: the epidermal component. Clin Dermatol. 2007;25:589–95.
Albanesi C, De Pità O, Girolomoni G. Resident skin cells in psoriasis: a special look at the pathogenetic functions of keratinocytes. Clin Dermatol. 2007;25:581–8.
Gaspari A. Innate and adaptive immunity and the pathophysiology of psoriasis. J Am Acad Dermatol. 2006;54:S67–80.
Gerritsen MJP. Dithranol. In: van de Kerkhof P, editor. Textbook of Psoriasis. Oxford: Blackwell; 2003. p. 170–85.
Müller K, Leukel P, Mayer KK, Wiegrebe W. Modification of DNA bases by anthralin and related compounds. Biochem Pharmacol. 1995;49:1607–13.
McGill A, Frank A, Emmett N, Leech SN, Turnbull DM, Birch-Machin MA, Reynolds NJ. The antipsoriatic drug anthralin accumulates in keratinocyte mitochondria, dissipates mitochondrial membrane potential, and induces apoptosis through a pathway dependent on respiratory competent mitochondria. FASEB J. 2005;04-2664.
Peus D, Beyerle A, Vasa M, Pott M, Meves A, Pittelkow MR. Antipsoriatic drug anthralin induces EGF receptor phosphorylation in keratinocytes: requirement for H2O2 generation. Exp Dermatol. 2004;13:78–85.
Reichert U, Jacques Y, Grangeret M, Schmidt R. Antirespiratory and antiproliferative activity of anthralin in cultured human keratinocytes. J Invest Dermatol. 1985;84:130–4.
Thomaand K, Holzmann C. Photostability of dithranol. Eur J Pharm Biopharm. 1998;46:201–8.
DiSepio D, Chandraratna RAS, Nagpal S. Novel approaches for the treatment of psoriasis. Drug Discov Today. 1999;4:222–31.
Menterand A, Griffiths CEM. Current and future management of psoriasis. Lancet. 2007;370:272–84.
Lau WM, White AW, Gallagher SJ, Donaldson M, McNaughton G, Heard CM. Scope and limitations of the co-drug approach to topical drug delivery. Curr Pharm Des. 2008;14:794–802.
Bonina FP, Puglia C, Barbuzzi T, de Caprariis P, Palagiano F, Rimoli MG, et al. In vitro and in vivo evaluation of polyoxyethylene esters as dermal prodrugs of ketoprofen, naproxen and diclofenac. Eur J Pharm Sci. 2001;14:123–34.
Gillard SE, Finlay AY. Current management of psoriasis in the United Kingdom: patterns of prescribing and resource use in primary care. Int J Clin Pract. 2005;59:1260–7.
Lau WM. Improved topical therapeutic systems based on co-drugs. Welsh School of Pharmacy. PhD thesis. 2008.
International Conference of Harmonisation. Guidance for industry: Q1A(R2) stability testing of new drug substances and products. www.fda.gov/CbER/gdlns/ichstab.htm (accessed 12 Apr 2008).
Sekkat N, Kalia YN, Guy RH. Porcine ear skin as a model for the assessment of transdermal drug delivery to premature neonates. Pharm Res. 2004;21:1390–7.
Thomas CP, Heard CM. Probing the skin permeation of eicosapentaenoic acid and ketoprofen: 2. Comparative depth profiling and metabolism of eicosapentaenoic acid. Eur J Pharm Biopharm. 2007;67:156–65.
Vallet V, Cruz C, Josse D, Bazire A, Lallement G, Boudry I. In vitro percutaneous penetration of organophosphorus compounds using full-thickness and split-thickness pig and human skin. Toxicol In Vitro. 2007;21:1182–90.
Simonand GA, Maibach HI. The pig as an experimental animal model of percutaneous permeation in man: qualitative and quantitative observations—an overview. Skin Pharmacol Appl Skin Physiol. 2000;13:229–34.
Meyer W, Kacza J, Zschemisch N-H, Godynicki S, Seeger J. Observations on the actual structural conditions in the stratum superficiale dermidis of porcine ear skin, with special reference to its use as model for human skin. Annals of Anatomy—Anatomischer Anzeiger. 2007;189:143–56.
Schmook FP, Meingassner JG, Billich A. Comparison of human skin or epidermis models with human and animal skin in in-vitro percutaneous absorption. Int J Pharm. 2001;215:51–6.
Bos JD, Meinardi MMHM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9:165–9.
Willson RJ. A thermodynamic exploration into pharmaceutical drug solubility. Drug Discov Today. 2001;6:985–6.
Leung DYM, Szfler SJ, Noman PS, Apter A, Eichenfield LF, Beck L. Elidel (pimecrolimus) cream 1%: A nonsteroidal topical agent for the treatment of atopic dermatitis. J Allergy Clin Immunol. 2003;111:1154–68.
Alaiti S, Kang S, Fiedler VC, Ellis CN, Spurlin DV, Fader D, et al. Tacrolimus (FK506) ointment for atopic dermatitis: A phase I study in adults and children. J Am Acad Dermatol. 1998;38:69–76.
The British Pharmacopoeia Commission. British Pharmacopoeia 2008 The Stationery Office, London, 2007.
Arellano A, Santoyo S, Martin C, Ygartua P. Influence of propylene glycol and isopropyl myristate on the in vitro percutaneous penetration of diclofenac sodium from carbopol gels. Eur J Pharm Sci. 1999;7:129–35.
Goldberg-Cettina M, Liu P, Nightingale J, Kurihara-Bergstrom T. Enhanced transdermal delivery of estradiol in vitro using binary vehicles of isopropyl myristate and short-chain alkanols. Int J Pharm. 1995;114:237–45.
Bealland HD, Sloan KB. Topical delivery of 5-fluorouracil (5-Fu) by 3-alkylcarbonyl-5-Fu prodrugs. Int J Pharm. 2001;217:127–37.
Mahrle G. Dithranol. Clin Dermatol. 1997;15:723–37.
Sasaki H, Takahashi T, Mori Y, Nakamura J, Shibasaki J. Transdermal delivery of 5-fluorouracil and its alkylcarbamoyl derivatives. Int J Pharm. 1990;60:1–9.
Wang JJ, Sung KC, Huang JF, Yeh CH, Fang JY. Ester prodrugs of morphine improve transdermal drug delivery: a mechanistic study. J Pharm Pharmacol. 2007;59:917–25.
Bickers DR, Dutta-Choudhury T, Mukhtar H. Epidermis: a site of drug metabolism in neonatal rat skin. Studies on cytochrome P-450 content and mixed-function oxidase and epoxide hydrolase activity. Mol Pharmacol. 1982;21:239–47.
Das M, Bickers DR, Mukhtar H. Epidermis: the major site of cutaneous benzo(a)pyrene and benzo(a)pyrene 7, 8-diol metabolism in neonatal BALB/c mice. Drug Metab Disposition. 1986;14:637–42.
Ziboh VA, Miller CC, Cho Y. Metabolism of polyunsaturated fatty acids by skin epidermal enzymes: generation of antiinflammatory and antiproliferative metabolites. Am J Clin Nutr. 2000;71:361S–6S.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the financial support from Stiefel Laboratories, UK.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lau, W.M., White, A.W. & Heard, C.M. Topical Delivery of a Naproxen-Dithranol Co-drug: In Vitro Skin Penetration, Permeation, and Staining. Pharm Res 27, 2734–2742 (2010). https://doi.org/10.1007/s11095-010-0274-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-010-0274-8