Abstract
Purpose
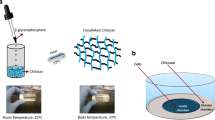
To determine the in vitro sub-cellular localization and in vivo efficacy of chitosan/GMO nanostructures containing paclitaxel (PTX) compared to a conventional PTX treatment (Taxol®).
Methods
The sub-cellular localization of coumarin-6 labeled chitosan/GMO nanostructures was determined by confocal microscopy in MDA-MB-231 cells. The antitumor efficacy was evaluated in two separate studies using FOX-Chase (CB17) SCID Female-Mice MDA-MB-231 xenograph model. Treatments consisted of intravenous Taxol® or chitosan/GMO nanostructures with or without PTX, local intra-tumor bolus of Taxol® or chitosan/GMO nanostructures with or without PTX. The tumor diameter and animal weight was monitored at various intervals. Histopathological changes were evaluated in end-point tumors.
Results
The tumor diameter increased at a constant rate for all the groups between days 7-14. After a single intratumoral bolus dose of chitosan/GMO containing PTX showed significant reduction in tumor diameter on day 15 when compared to control, placebo and intravenous PTX administration. The tumor diameter reached a maximal decrease (4-fold) by day 18, and the difference was reduced to approximately 2-fold by day 21. Qualitatively similar results were observed in a separate study containing PTX when administered intravenously.
Conclusion
Chitosan/GMO nanostructures containing PTX are safe and effective administered locally or intravenously. Partially supported by DOD Award BC045664








Similar content being viewed by others
REFERENCES
Singla AK, Garg A, Aggarwal GD. Paclitaxel and its formulations. Int J Pharm. 2002;235:179–92. doi:10.1016/S0378-5173(01)00986-3.
Tarr BD, Yalkowsky SH. A new parenteral vehicle for the administration of some poorly soluble anti-cancer drugs. J Parenter Sci Technol. 1987;41:31–33.
Chao TC, Chu Z, Tseng LM, Chiou TJ, Hsieh RK, Wang WS, et al. Paclitaxel in a novel formulation containing less Cremophor EL as first-line therapy for advanced breast cancer: a phase II trial. Invest New Drugs. 2005;23:171–7. doi:10.1007/s10637-005-5863-8.
Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37:1590–8. doi:10.1016/S0959-8049(01)00171-X.
Friedland D, Gorman G, Treat J. Hypersensitivity reactions from taxol and etoposide. J Natl Cancer Inst. 1993;85:2036. doi:10.1093/jnci/85.24.2036.
Rowinsky EK, Chaudhry V, Cornblath DR, Donehower RC. Neurotoxicity of Taxol. J Natl Cancer Inst Monogr. 1993;15:107–15.
Ibrahim NK, Desai N, Legha S, Soon-Shiong P, Theriault RL, Rivera E, et al. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002;8:1038–44.
Gradishar WJ. Albumin-bound paclitaxel: a next-generation taxane. Expert Opin Pharmacother. 2006;7:1041–53. doi:10.1517/14656566.7.8.1041.
Socinski M. Update on nanoparticle albumin-bound paclitaxel. Clin Adv Hematol Oncol. 2006;4:745–6.
Lee Villano J, Mehta D, Radhakrishnan L. Abraxane induced life-threatening toxicities with metastatic breast cancer and hepatic insufficiency. Invest New Drugs. 2006;24:455–6. doi:10.1007/s10637-006-6214-0.
Moore A, Medarova Z, Potthast A, Dai G. In vivo targeting of underglycosylated MUC-1 tumor antigen using a multimodal imaging probe. Cancer Res. 2004;64:1821–7. doi:10.1158/0008-5472.CAN-03-3230.
Sandri G, Bonferoni MC, Rossi S, Ferrari F, Gibin S, Zambito Y, et al. Nanoparticles based on N-trimethylchitosan: evaluation of absorption properties using in vitro (Caco-2 cells) and ex vivo (excised rat jejunum) models. Eur J Pharm Biopharm. 2007;65:68–77. doi:doi:10.1016/j.ejpb.2006.07.016.
Bernkop-Schnurch A, Weithaler A, Albrecht K, Greimel A. Thiomers: preparation and in vitro evaluation of a mucoadhesive nanoparticulate drug delivery system. Int J Pharm. 2006;317:76–81. doi:10.1016/j.ijpharm.2006.02.044.
Cui F, Qian F, Yin C. Preparation and characterization of mucoadhesive polymer-coated nanoparticles. Int J Pharm. 2006;316:154–61. doi:10.1016/j.ijpharm.2006.02.031.
Howard KA, Rahbek UL, Liu X, Damgaard CK, Glud SZ, Andersen MO, et al. RNA interference in vitro and in vivo using a novel chitosan/siRNA nanoparticle system. Mol Ther. 2006;14:476–84. doi:10.1016/j.ymthe.2006.04.010.
Zheng F, Shi XW, Yang GF, Gong LL, Yuan HY, Cui YJ, et al. Chitosan nanoparticle as gene therapy vector via gastrointestinal mucosa administration: results of an in vitro and in vivo study. Life Sci. 2007;80:388–96. doi:10.1016/j.lfs.2006.09.040.
Takeuchi H, Yamamoto H, Niwa T, Hino T, Kawashima Y. Enteral absorption of insulin in rats from mucoadhesive chitosan-coated liposomes. Pharm Res. 1996;13:896–901. doi:10.1023/A:1016009313548.
Takeuchi H, Thongborisute J, Matsui Y, Sugihara H, Yamamoto H, Kawashima Y. Novel mucoadhesion tests for polymers and polymer-coated particles to design optimal mucoadhesive drug delivery systems. Adv Drug Deliv Rev. 2005;57:1583–94. doi:10.1016/j.addr.2005.07.008.
Thongborisute J, Tsuruta A, Kawabata Y, Takeuchi H. The effect of particle structure of chitosan-coated liposomes and type of chitosan on oral delivery of calcitonin. J Drug Target. 2006;14:147–54. doi:10.1080/10611860600648346.
Trickler WJ, Nagvekar AA, Dash AK. A Novel Nanoparticle Formulation for Sustained Paclitaxel Delivery. AAPS PharmSciTech. 2008;9:486–93. doi:10.1208/s12249-008-9063-7.
Davda J, Labhasetwar V. Characterization of nanoparticle uptake by endothelial cells. Int J Pharm. 2002;233:51–9. doi:10.1016/S0378-5173(01)00923-1.
Shikata F, Tokumitsu H, Ichikawa H, Fukumori Y. In vitro cellular accumulation of gadolinium incorporated into chitosan nanoparticles designed for neutron-capture therapy of cancer. Eur J Pharm Biopharm. 2002;53:57–63. doi:10.1016/S0939-6411(01)00198-9.
Xu ZP, Niebert M, Porazik K, Walker TL, Cooper HM, Middelberg AP, et al. Subcellular compartment targeting of layered double hydroxide nanoparticles. J Control Release. 2008;130:86–94. doi:10.1016/j.jconrel.2008.05.021.
Yin M, Shen J, Gropeanu R, Pflugfelder GO, Weil T, Mullen K. Fluorescent core/shell nanoparticles for specific cell-nucleus staining. Small. 2008;4:894–8. doi:10.1002/smll.200701107.
Perumal OP, Inapagolla R, Kannan S, Kannan RM. The effect of surface functionality on cellular trafficking of dendrimers. Biomaterials. 2008;29:3469–76. doi:10.1016/j.biomaterials.2008.04.038.
Zou J, Saulnier P, Perrier T, Zhang Y, Manninen T, Toppila E, et al. Distribution of lipid nanocapsules in different cochlear cell populations after round window membrane permeation. J Biomed Mater Res B Appl Biomater. 2008;87:10–8.
de la Fuente M, Seijoand B, Alonso MJ. Bioadhesive hyaluronan-chitosan nanoparticles can transport genes across the ocular mucosa and transfect ocular tissue. Gene Ther. 2008;15:668–76. doi:10.1038/gt.2008.16.
Lacoeuille F, Garcion E, Benoit JP, Lamprecht A. Lipid nanocapsules for intracellular drug delivery of anticancer drugs. J Nanosci Nanotechnol. 2007;7:4612–7.
Zhang LW, Yu WW, Colvin VL, Monteiro-Riviere NA. Biological interactions of quantum dot nanoparticles in skin and in human epidermal keratinocytes. Toxicol Appl Pharmacol. 2008;228:200–11. doi:10.1016/j.taap. 2007.12.022.
Panyam J, Labhasetwar V. Targeting intracellular targets. Curr Drug Deliv. 2004;1:235–47. doi:10.2174/1567201043334768.
Panyam J, Zhou WZ, Prabha S, Sahoo SK, Labhasetwar V. Rapid endo-lysosomal escape of poly(DL-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. Faseb J. 2002;16:1217–26. doi:10.1096/fj.02-0088com.
Erni C, Suard C, Freitas S, Dreher D, Merkle HP, Walter E. Evaluation of cationic solid lipid microparticles as synthetic carriers for the targeted delivery of macromolecules to phagocytic antigen-presenting cells. Biomaterials. 2002;23:4667–76. doi:10.1016/S0142-9612(02)00216-8.
Koval M, Preiter K, Adles C, Stahl PD, Steinberg TH. Size of IgG-opsonized particles determines macrophage response during internalization. Exp Cell Res. 1998;242:265–73. doi:10.1006/excr.1998.4110.
Costanzo PJ, Patten TE, Seery TA. Nanoparticle agglutination: acceleration of aggregation rates and broadening of the analyte concentration range using mixtures of various-sized nanoparticles. Langmuir. 2006;22:2788–94. doi:10.1021/la0522909.
Chavanpatil MD, Khdair A, Panyam J. Nanoparticles for cellular drug delivery: mechanisms and factors influencing delivery. J Nanosci Nanotechnol. 2006;6:2651–63. doi:10.1166/jnn.2006.443.
Panyam J, Labhasetwar V. Dynamics of endocytosis and exocytosis of poly(D, L-lactide-co-glycolide) nanoparticles in vascular smooth muscle cells. Pharm Res. 2003;20:212–20. doi:10.1023/A:1022219003551.
Harush-Frenkel O, Rozentur E, Benita S, Altschuler Y. Surface charge of nanoparticles determines their endocytic and transcytotic pathway in polarized MDCK cells. Biomacromolecules. 2008;9:435–43. doi:10.1021/bm700535p.
Harush-Frenkel O, Debotton N, Benita S, Altschuler Y. Targeting of nanoparticles to the clathrin-mediated endocytic pathway. Biochem Biophys Res Commun. 2007;353:26–32. doi:10.1016/j.bbrc.2006.11.135.
Xu P, Van Kirk EA, Zhan Y, Murdoch WJ, Radosz M, Shen Y. Targeted charge-reversal nanoparticles for nuclear drug delivery. Angew Chem Int Ed Engl. 2007;46:4999–5002. doi:10.1002/anie.200605254.
Berry CC, de la Fuente JM, Mullin M, Chu SW, Curtis AS. Nuclear localization of HIV-1 tat functionalized gold nanoparticles. IEEE Trans Nanobioscience. 2007;6:262–9. doi:10.1109/TNB.2007.908973.
Ryan JA, Overton KW, Speight ME, Oldenburg CN, Loo L, Robarge W, et al. Cellular uptake of gold nanoparticles passivated with BSA-SV40 large T antigen conjugates. Anal Chem. 2007;79:9150–9. doi:10.1021/ac0715524.
Tkachenko AG, Xie H, Liu Y, Coleman D, Ryan J, Glomm WR, et al. Cellular trajectories of peptide-modified gold particle complexes: comparison of nuclear localization signals and peptide transduction domains. Bioconjug Chem. 2004;15:482–90. doi:10.1021/bc034189q.
Tkachenko AG, Xie H, Coleman D, Glomm W, Ryan J, Anderson MF, et al. Multifunctional gold nanoparticle-peptide complexes for nuclear targeting. J Am Chem Soc. 2003;125:4700–1. doi:10.1021/ja0296935.
Mugabe C, Hadaschik BA, Kainthan RK, Brooks DE, So AI, Gleave ME, Burt HM. Paclitaxel incorporated in hydrophobically derivatized hyperbranched polyglycerols for intravesical bladder cancer therapy. BJU Int (2008)
Danhier F, Lecouturier N, Vroman B, Jerome C, Marchand-Brynaert J, Feron O, et al. Paclitaxel-loaded PEGylated PLGA-based nanoparticles: in vitro and in vivo evaluation. J Control Release. 2009;133:11–7. doi:10.1016/j.jconrel.2008.09.086.
Zhang Z, Lee SH, Gan CW, Feng SS. In vitro and in vivo investigation on PLA-TPGS nanoparticles for controlled and sustained small molecule chemotherapy. Pharm Res. 2008;25:1925–35. doi:10.1007/s11095-008-9611-6.
Nornoo AO, Chow DS. Cremophor-free intravenous microemulsions for paclitaxel II. Stability, in vitro release and pharmacokinetics. Int J Pharm. 2008;349:117–23. doi:10.1016/j.ijpharm.2007.07.043.
Dong Y, Feng SS. In vitro and in vivo evaluation of methoxy polyethylene glycol-polylactide (MPEG-PLA) nanoparticles for small-molecule drug chemotherapy. Biomaterials. 2007;28:4154–60. doi:10.1016/j.biomaterials.2007.05.026.
Lee SW, Chang DH, Shim MS, Kim BO, Kim SO, Seo MH. Ionically fixed polymeric nanoparticles as a novel drug carrier. Pharm Res. 2007;24:1508–16. doi:10.1007/s11095-007-9269-5.
Koziara JM, Whisman TR, Tseng MT, Mumper RJ. In-vivo efficacy of novel paclitaxel nanoparticles in paclitaxel-resistant human colorectal tumors. J Control Release. 2006;112:312–9. doi:10.1016/j.jconrel.2006.03.001.
Win KY, Feng SS. In vitro and in vivo studies on vitamin E TPGS-emulsified poly(D, L-lactic-co-glycolic acid) nanoparticles for paclitaxel formulation. Biomaterials. 2006;27:2285–91. doi:10.1016/j.biomaterials.2005.11.008.
Chen DB, Yang TZ, Lu WL, Zhang Q. In vitro and in vivo study of two types of long-circulating solid lipid nanoparticles containing paclitaxel. Chem Pharm Bull (Tokyo). 2001;49:1444–7. doi:10.1248/cpb.49.1444.
Sharma D, Chelvi TP, Kaur J, Chakravorty K, De TK, Maitra A, et al. Novel Taxol formulation: polyvinylpyrrolidone nanoparticle-encapsulated Taxol for drug delivery in cancer therapy. Oncol Res. 1996;8:281–6.
Wang Y, Li Y, Zhang L, Fang X. Pharmacokinetics and biodistribution of paclitaxel-loaded pluronic P105 polymeric micelles. Arch Pharm Res. 2008;31:530–8. doi:10.1007/s12272-001-1189-2.
Han LM, Guo J, Zhang LJ, Wang QS, Fang XL. Pharmacokinetics and biodistribution of polymeric micelles of paclitaxel with Pluronic P123. Acta Pharmacol Sin. 2006;27:747–53. doi:10.1111/j.1745-7254.2006.00340.x.
Kim SC, Kim DW, Shim YH, Bang JS, Oh HS, Wan Kim S, et al. In vivo evaluation of polymeric micellar paclitaxel formulation: toxicity and efficacy. J Control Release. 2001;72:191–202. doi:10.1016/S0168-3659(01)00275-9.
Hamaguchi T, Matsumura Y, Suzuki M, Shimizu K, Goda R, Nakamura I, et al. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Br J Cancer. 2005;92:1240–6. doi:10.1038/sj.bjc.6602479.
Yang T, Choi MK, Cui FD, Lee SJ, Chung SJ, Shim CK, et al. Antitumor effect of paclitaxel-loaded PEGylated immunoliposomes against human breast cancer cells. Pharm Res. 2007;24:2402–11. doi:10.1007/s11095-007-9425-y.
Turturro F, Von Burton G, Friday E. Hyperglycemia-induced thioredoxin-interacting protein expression differs in breast cancer-derived cells and regulates paclitaxel IC50. Clin Cancer Res. 2007;13:3724–30. doi:10.1158/1078-0432.CCR-07-0244.
Han GZ, Liu ZJ, Shimoi K, Zhu BT. Synergism between the anticancer actions of 2-methoxyestradiol and microtubule-disrupting agents in human breast cancer. Cancer Res. 2005;65:387–93.
ACKNOWLEDGEMENTS
This work was partially supported by a Department of Defense Concept Award BC045664 and Health Future Foundations, Omaha, NE. This research was also partially conducted at the Integrative Biological Imaging Facility at Creighton University, Omaha, NE. This facility, supported by the C.U. Medical School, was constructed with support from C06 Grant RR17417-01 from the NCRR, NIH.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trickler, W., Nagvekar, A. & Dash, A. The In vitro Sub-cellular Localization and In vivo Efficacy of Novel Chitosan/GMO Nanostructures containing Paclitaxel. Pharm Res 26, 1963–1973 (2009). https://doi.org/10.1007/s11095-009-9911-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-009-9911-5