ABSTRACT
Purpose
The dissolution of HPMC matrix tablets containing different amounts of highly soluble (mannitol) or poorly soluble (dicalcium phosphate, DCP) was studied to deduce the parameters critical to release robustness.
Methods
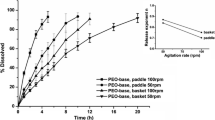
The release of HPMC and additives was studied using a modified USP II method at two paddle stirring rates, 50 and 125 rpm, at HPMC content varying from 15% to 100%.
Results
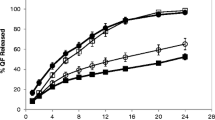
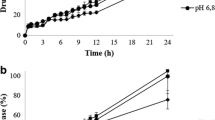
At HPMC contents between 30% and 35% a critical point was identified and found crucial to the release from the HPMC/mannitol tablets. Below this point the matrix rapidly disintegrated in a non robust manner. At higher HPMC contents the mannitol release became increasingly diffusion controlled with maintained matrix integrity. The release robustness was lower for HPMC/DCP than HPMC/mannitol tablets at high HPMC contents, however, lacking critical points. The critical point was interpreted as the percolation threshold for HPMC and differences explained in terms of water transport into the matrix.
Conclusion
The release robustness was lower for formulations with additives of low solubility having an erosion controlled release than for additives with higher solubility and a diffusion controlled release. However, for additives creating a steep osmotic pressure gradient, an HPMC content above the percolation threshold becomes vital for maintaining the release robustness.






Similar content being viewed by others
References
D.A. Alderman. A review of cellulose ethers in hydrophilic matrices for oral controlled-release dosage forms. Int J Pharm Tech Prod. 5:1–9 (1984).
T.D. Reynolds, S.H. Gehrke, A.S. Hussain, and S.L.S. Shenouda. Polymer erosion and drug release characterization of hydroxypropyl methylcellulose matrices. J Pharm Sci. 87:1115–1123 (1998) doi:10.1021/js980004q.
B. Abrahamsson, M. Alpsten, B. Bake, A. Larsson, and J. Sjögren. In vitro and in vivo erosion of two different hydrophilic gel matrix tablets. Eur J Pharm and Biopharm. 46:69–75 (1998) doi:10.1016/S0939-6411(98)00002-2.
K. Sako, T. Sawada, H. Nakashima, S. Yokohama, and T. Sonobe. Influence of water soluble fillers in hydroxypropylmethylcellulose matrices on in vitro and in vivo drug release. J Control Release. 81:165–172 (2002) doi:10.1016/S0168-3659(02)00067-6.
B. Abrahamsson, K. Roos, and J. Sjögren. Investigation of prandial effects on hydrophilic matrix tablets. Drug Dev and Ind Pharm. 25:765–771 (1999) doi:10.1081/DDC-100102236.
P. Colombo, R. Bettini, P. Santi, and A.N. Peppas. Swellable matrices for controlled drug delivery: gel-layer behaviour, mechanisms and optimal performance. Pharm Sci Technol Today. 3:198–204 (2000) doi:10.1016/S1461-5347(00)00269-8.
A.N. Peppas. Hydrogels in medicine and pharmacy, properties and applications, vol III. CRS, Boca Raton, Florida, 2000, pp. 27–56.
R.T. Ju, P.R. Nixon, and M.V. Patel. Drug release from hydrophilic matrices. 1. New scaling law for predicting polymer and drug release based on the polymer disentanglement concentration and the diffusion layer. J Pharm Sci. 84:1455–1463 (1995) doi:10.1002/jps.2600841213.
A. K, rner, A. Larsson, L. Piculell, and B. Wittgren. Molecular information on the dissolution of polydisperse polymers: mixtures of long and short poly(ethylene oxide). J Phys Chem. 109:11530–11537 (2005).
S. Jamzad, L. Tutunji, and R. Fassihi. Analysis of macromolecular changes and drug release from hydrophilic matrix systems. Int J Pharm. 292:75–85 (2005) doi:10.1016/j.ijpharm.2004.11.011.
H. Leuenberger, B.D. Rohera, and C. Haas. Percolation theory—a novel approach to solid dosage form design. Int J Pharm. 38:109–115 (1987) doi:10.1016/0378-5173(87)90105-0.
I. Fuertes, A. Miranda, M. Millán, and I. Caraballo. Estimation of the percolation thresholds in acyclovir hydrophilic matrix tablets. Eur J Pharm Biopharm. 64:336–342 (2006) doi:10.1016/j.ejpb.2006.05.009.
A. Miranda, M. Millán, and I. Caraballo. Study of the critical points of HPMC hydrophilic matrices for controlled drug delivery. Int J Pharm. 311:75–81 (2006) doi:10.1016/j.ijpharm.2005.12.012.
A. Miranda, M. Millán, and I. Caraballo. Investigation of the influence of particle size on the excipient percolation thresholds of HPMC hydrophilic matrix tablets. J Pharm Sci. 96:2746–2756 (2007) doi:10.1002/jps.20912.
T. Goncalves-Araújo, A.R. Rajabi-Siahboomi, and I. Caraballo. Application of percolation theory in the study of an extended release Verapamil hydrochloride formulation. Int J Pharm. 361:112–117 (2008) doi:10.1016/j.ijpharm.2008.05.022.
F. Tajarobi, S. Abrahmsén-Alami, A.S. Carlsson, and A. Larsson. Simultaneous probing of swelling, erosion and dissolution by NMR-microimaging—effect of solubility of additives on HPMC matrix tablets. Eur J Pharm Sci (2009). doi:10.1016/j.ejps.2009.01.008.
J. Johansson, S. Folestad, and B. Abrahamsson. Novel process analytical methodology to establish design space and real time prediction of dissolution. AAPS Workshop on Challenges for Dissolution Testing for the 21st Century, Arlington, VA, USA, May 1–3. (2006).
P. Timmins, A.M. Delargy, C.M. Minchom, and J.R. Howard. Influence of some process variables on product properties for a hydrophilic matrix controlled release tablet. Eur J Pharm Biopharm. 38:113–118 (1992).
M.V. Velasco, J.L. Ford, P. Rowe, and A.R. Rajabi-Siahhoomi. Influence of drug:hydroxypropyl methyl cellulose ratio, drug and polymer particle size and compression force on the release of diclofenac sodium from HPMC tablets. J Control Release. 57:75–85 (1999) doi:10.1016/S0168-3659(98)00110-2.
M. Levinaand, and A.R. Rajabi-Siahboomi. The influence of excipients on drug release from hydroxypropyl methylcellulose matrices. J Pharm Sci. 93:2746–2754 (2004) doi:10.1002/jps.20181.
Acknowledgments
The authors acknowledge AstraZeneca and Swedish Knowledge Foundation (particularly the YPK-project) for funding this work. Sincere thanks to Anders S. Carlsson and Jonas Johansson at AstraZeneca for their advisory and technical assistance with the analytical methods (LC-MS) and FBRM-technology, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tajarobi, F., Abrahmsén-Alami, S., Hansen, M. et al. The Impact of Dose and Solubility of Additives on the Release from HPMC Matrix Tablets—Identifying Critical Conditions. Pharm Res 26, 1496–1503 (2009). https://doi.org/10.1007/s11095-009-9861-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-009-9861-y