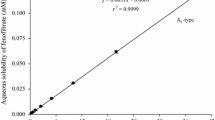
Abstract
Purpose
The aim of this study is to develop a new process for manufacturing a nano-sized form of the popular cholesterol-reducing drug fenofibrate which can be implemented on industrial scale with minimal changes of currently used production schemes.
Methods
Salt-assisted milling was used to reduce particle size of commercial fenofibrate from micron-sized particles to nanometer domains.
Results
The optimal parameters for the salt milling are reported, allowing one to reduce the particle size from tens of micrometers to a hundred of nanometers. Dissolution of nano-sized fenofibrate was studied in various formulations and compared against the micron-sized commercially available fenofibrate.
Conclusions
The nano-sized fenofibrate demonstrates faster dissolution kinetics in aqueous media, simulating stomach environment, within the first 60 min as compared to the micronized form. The highest dissolution rate is achieved with the nano-sized fenofibrate when surfactants, such as sodium dodecyl sulfate or inclusion complex forming agents such as alpha-cyclodextrin, are used.







Similar content being viewed by others
References
G. L. Amidon, H. Lennernas, V. P. Shah et al. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 12(3):413–420 (1995) doi:10.1023/A:1016212804288.
R. D. Connors, and E. J. Elder. Using a portfolio of particle growth technologies to enable delivery of drugs with poor water solubility. Drug Deliv. Technol. 4(8):78–84 (2004).
E. Merisko-Liversidge, G. G. Liversidge, and E. R. Cooper. Nanosizing: a formulation approach for poorly-water-soluble compounds. Eur. J. Pharm. Sci. 18(2):113–120 (2003) doi:10.1016/S0928-0987(02)00251-8.
A. P. Tinke, K. Vanhoutte, R. De Maesschalck et al. A new approach in the prediction of the dissolution behavior of suspended particles by means of their particle size distribution. J. Pharm. Biomed. Anal. 39(5):900–907 (2005) doi:10.1016/j.jpba.2005.05.014.
H. M. Deng, J. Ding, Y. Shi et al. Ultrafine zinc oxide prepared by precipitation/mechanical milling. J.Mater. Sci. 36(13):3273–3276 (2001) doi:10.1023/A:1017902923289.
E. Reverchon, and G. Della Porta. Supercritical fluids-assisted micronization techniques. Low-impact routes for particle production. Pure Appl. Chem. 73(8):1293–1297 (2001) doi:10.1351/pac200173081293.
H. Sucker, and P. Gassmann. Improvements in pharmaceutical compositions. GB Patent 2269536A, 1994
J. Shepherd. Mechanism of action of fibrates. Postgrad. Med. J. 69(Suppl. 1):S34–S41 (1993) doi:10.1136/pgmj.69.807.80.
S. Jamzad, and R. Fassihi. Role of surfactant and pH on dissolution properties of fenofibrate and glipizide—a technical note. AAPS Pharm. Sci. Tech. 7(2):Article 33 (2006) doi:10.1208/pt070233.
A. Munoz, J. P. Guichard, and P. Reginault. Micronised fenofibrate. Atherosclerosis. 110(Suppl):S45–S48 (1994) doi:10.1016/0021-9150(94)05375-S.
C. Mochales, H. El Briak-BenAbdeslam, M. P. Ginebra et al. Dry mechanochemical synthesis of hydroxyapatites from DCPD and CaO: influence of instrumental parameters on the reaction kinetics. Biomaterials. 25(7–8):1151–1158 (2003) doi:10.1016/j.biomaterials.2003.08.002.
C. Noory, N. Tran, L. Ouderkirk et al. Steps for development of a dissolution test for sparingly water-soluble drug products. Am. Pharm. Rev. 5(4):16–21 (2002).
Acknowledgements
This project was conducted under NSF grant CMMI-0556082. SEM and XRD were operated by the Centralized Research Facility of the College of Engineering, Drexel University. The authors wish to thank iCeutica Inc., Dr. Matt Callahan, Dr. Raff Cammarano, and Dr. Felix Meiser for useful technical discussions and recommendations during the manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mochalin, V.N., Sagar, A., Gour, S. et al. Manufacturing Nanosized Fenofibrate by Salt Assisted Milling. Pharm Res 26, 1365–1370 (2009). https://doi.org/10.1007/s11095-009-9846-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-009-9846-x