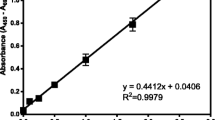
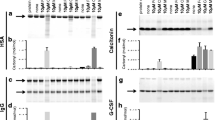
Abstract
Purpose
L-histidine, a commonly used buffer for protein formulations, has the potential to oxidize and form multiple byproducts. Previous studies were performed using metal catalyzed oxidation with Fe2+ or Cu2+. We re-examined the oxidation of L-histidine under conditions more appropriate to protein formulations.
Methods
Solutions of free L-histidine, protected from light, were initially reacted with tert-butylhydroperoxide and the products analyzed by UV absorption spectroscopy, reversed phase HPLC and mass spectrometric analysis and NMR. Experimental parameters investigated were oxidizing agent, pH, temperature, metal ion and metal chelator content.
Results
The initial reaction produced a number of known products, along with an unknown product that was identified as 4(5)-imidazolecarboxaldehyde. The reaction was highly pH and oxidizing-agent specific. The product was not observed at pH 5.0 or below, while there was a dramatic increase for reactions carried out at pH 6.0 or above. Addition of FeSO4 to the reaction dramatically increased the amount of 4(5)-imidazolecarboxaldehyde produced, while addition of the metal chelators EDTA or DTPA completely inhibited the reaction.
Conclusions
The presence of oxidants and trace concentrations of metal ions in high purity L-histidine solutions results in the formation of 4(5)-imidazolecarboxaldehyde which has the potential to covalently modify proteins.












Similar content being viewed by others
References
Chen B, Bautista R, Yu K, Zapata GA, Mulkerrin MG, Chamow SM. Influence of histidine on the stability and physical properties of a fully human antibody in aqueous and solid forms. Pharm Res. 2003;20(12):1952–60.
Miskoski S, Garcia NA. Influence of the peptide bond on the singlet molecular oxygen-mediated (O2[delta g]) photooxidation of histidine and methionini depeptides. A kinetic Study. Photoch Photobiol. 1993;57(3):447–52.
Wasserman HH, Wolff MS, Stiller K. The dye-sensitized photooxidation of imidazoles—trapping of intermediates by nucleophiles. Tetrahedron. 1980;37(1):191–200.
Iesce MR, Cermola F, Temussi F. Photooxygenation of heterocycles. Curr Org Chem. 2005;9(2):109–39.
George MV, Bhat B. Photooxygenation of nitrogen heterocycles. Chem Rev. 1979;79(5):447–78.
Davies MJ, Truscott RJW. Photo-oxidation of proteins and its role in cataractogenesis. J Photochem Photobiol B: Biol. 2001;63:114–25.
Kang P, Foote CS. Photosensitized oxidation of 13C, 15N-labeled imidazole derivatives. J Am Chem Soc. 2002;124(32):9629–38.
Tomita M, Irie M, Ukita T. Sensitized photooxidation of histidine and its derivatives. Products and mechanism of the reaction. Biochemistry. 1969;8(12):5149–60.
Dakin HD. The oxidation of amido-acids with the production of substances of biological importance. J Biol Chem. 1906;1(2):171–6.
Dakin HD. The formation of glyoxylic acid. J Biol Chem. 1906;1(4):271–8.
Dakin HD. The Oxidation of Leucin, α-amido-isovaleric acid and of α-amido-n-valeric acid with hydrogen peroxide. The J Biol Chem. 1908;4(1):63–76.
Amici A, Levine RL, Tsai L, Stadtman ER. Conversion of amino acid residues in proteins and amino acid homopolymers to carbonyl derivatives by metal-catalyzed oxidation reactions. J Biol Chem. 1989;264(6):3341–6.
Stadtman ER. Oxidation of free amino acids and amino acid residues in proteins by radioylsis and by metal-catalyzed reactions. Ann Rev Biochem. 1993;62:797–821.
Stadtman ER, Berlett BS. Fenton chemistry. Amino acid oxidation. J Biol Chem. 1991;266(26):17201–11.
Uchida K, Kawakishi S. Identification of oxidized histidine generated at the active site of Cu, Zn-superoxide dismutase exposed to H2O2. Selective generation of 2- oxo-histidine at the histidine 118. J Biol Chem. 1994;269(4):2405–10.
Schoneich C. Mechanism of Metal-catalyzed oxidation of histidine to 2-oxo-histidine in peptides and proteins. J Pharm Biomed Anal. 2000;21:1093–7.
Gangopadhyay S, Ali M, Banerjee P. Kinetics and Mechanism of the Oxidation of Histidine by Dodecatungstocobaltate (III) and trans-Cyclohexane-1, 2-diamine-N, N, N′, N′-tetraacetatomanganate(III) in Aqueous Medium. J Chem Soc Perkin Trans. 1992;2(5):781–5.
Laloo D, Mahanti MK. Kinetics of oxidation of amino acids by alkaline hexacyanoferrate (III). J Chem Soc Dalt Trans. 1990;1990(1):311–3.
Hureiki L, Croue JP, Legube B. Chlorination studies of free and combined amino acids. Water Res. 1994;28(12):2521–31.
Pereira WE, Hoyano Y, Summons RE, Bacon VA, Duffield AM. Chlorination Studies II. The reaction of aqueous hypochlorous acid with α-amino acids and dipeptides. Biochim Biophys Acta. 1973;313:170–80.
Rangappa KS, Chandraju S, Gowda NMM. Manganese(III) oxidation of L-Lysine and L-Histidine in pyrophosphate solution: a kinetic and mechanistic study. Synth React Inorg Met-Org Chem. 1998;28(2):275–94.
Pinto I, Sherigara BS, Udupa HVK. Electrolytically generated manganese(III) sulfate for the oxidation of L-Histidine in aquoeous sulfuric acid: a kinetic study. Bull Chem Soc Jpn. 1990;63:3625–31.
Yong SH, Karel M. Reaction of histidine with methyl linoleate: characterization of the histidine degradation products. J Amer Oil Chem Soc. 1978;55:352–7.
Karim E, Mahanti MK. Kinetics of oxidation of amino acids by quinolinium dichromate. Pol J Chem. 1992;66:1471–6.
Satyanarayana MV, Sundar BS, Murti PSR. Kinetics and mechanism of oxidation of a few α-amino acids by trichloroisocyanuric acid. Oxid Comm. 1993;16(4):364–72.
Jones MN. Surfactants in membrane solubilisation. Int J Pharm. 1999;177:137–59.
Sivars U, Tjerneld F. Mechanisms of phase behaviour and protein partitioning in detergent/polymer aqueous two-phase systems for purification of integral membrane proteins. Biochim Biophys Acta. 2000;1474:133–46.
Ha E, Wang W, Wang YJ. Peroxide formation in polysorbate 80 and protein stability. J Pharm Sci. 2002;91(10):2252–64.
Cleland JL, Powell MF, Shire SJ. The development of stable protein formulations: a close look at protein aggregation, deamidation, and oxidation. Crit Rev Ther Drug Car Sys. 1993;10(4):307–77.
Carpenter JF, Pikal MJ, Chang BS, Randolph TW. Rational design of stable lyophilized protein formulations: some practical advice. Pharm Res. 1997;14(8):969–75.
Kappelgaard A-M. Liquid growth hormone: perservatives and buffers. Horm Res. 2004;62(S 3):98–103.
Katayama DS, Nayar R, Chou DK, Valente JJ, Cooper J, Henry CS, et al. Effect of buffer species on the thermally induced aggregation of interferon-tau. J Pharm Sci. 2006;95(6):1212–26.
Barnard ML, Gurdian S, Diep D, Ladd M, Turrens JF. Protein and amino acid oxidation is associated with increased chemiluminescence. Arch Biochem Biophys. 1993;300(2):651–6.
Auerbach PSN, Robert L. Harrison’s principles of internal medicine. 17th ed.: McGraw-Hill, 2008. p 2747.
Bertrand R, Capony J-P, Derancourt J, Kassab R. Detection of nucleotide- and F-actin induced movements in the switch II Helix of the skeletal myosin using its differential oxidative mediated by an iron-EDTA complex disulfide-linked to the strong actin binding site. Biochemistry. 1999;38:11914–25.
McCord JM, Day Jr ED. Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978;86(1):139–42.
Gewitz H-S, Piefke J, Langowska K, Vennesland B. The formation of hydrogen cyanide from histidine in the presence of amino acid oxidase and peroxidase. Biochim Biophys Acta (BBA) Enzymol. 1980;611(1):11–26.
Ahond A, Mourabit AA, Bedoya-Zurita M, Heng R, Braga RM, Poupat C, et al. Synthese stereoselective de la girolline. Tetrahedron. 1992;48(21):4327–46.
Aulaskari P, Ahlgren M, Rouvinen J, Vainiotalo P. Preparation and structure determination of 1-Benzyl-, 1-Methyl- and 1H-5-[(2-Nitro-2-Phenyl)ethenyl)imidazoles. J Heterocycl Chem. 1996;33:1345–54.
Kim J-W, Abdelaal SM, Bauer L, Heimer NE. Synthesis of 1-(Dimethylsulfamoyl)-2- and 5-Imidazolecarboxaldehydes. Rearrangement of 1-(Dimethylsulfamoyl)-5-imidazolecarboxaldehyde to the 4-Carboxaldehyde [1]. J Heterocycl Chem. 1995;32:611–20.
Kirk KL. 4-Lithio-l-tritylimidazole as a synthetic intermediate. Synthesis of imidazole-4-carboxaldehyde. J Heterocycl Chem. 1985;22:57–9.
Lindgren G, Stensio K-E, Wahlberg K. Synthesis and photocyclization of some 4-(5)Arylethenylimidazoles. J Heterocycl Chem. 1980;17:679–83.
Mohammad T, Kasper A, Morrison H. Urocanic acid photobiology. Purine assisted photooxidation to 1H-Imidazole-4(5)-Carboxaldehyde. Tetrahedron Lett. 1994;35(28):4903–6.
Papadopoulos EP, Jarrar A, Issidorides CH. Oxidations with manganese dioxide. J Org Chem. 1966;31(2):615–6.
Pyman FL. Derivatives of glyoxaline-4(or 5)-formaldehyde and glyoxaline-4(or 5)-carboxylic acid. A new synthesis of histidine. J Chem Soc Trans. 1916;109(197):186–202.
Reiter LA. Synthesis of 4(5)-Acyl-, 1-Substituted 5-Acyl-, and 1-Substituted 4-Acyl-1H-imidazoles from 4-Aminoisoxazoles. J Org Chem. 1986;52:2714–26.
Rosenberg H, Paul AG. Biosynthetic production of aberrant alkaloids in Dolichothele sphaerica (Cactaceae). J Pharm Sci. 1973;62(3):403–7.
Su Q, Wood JL. Regioselective N-Alkylation of 4-Formylimidazole. Synth Commun. 2000;30(18):3383–9.
Winter J, Retey J. A simple and efficient synthesis of N-Protected imidazole-4-carbaldehyde. Synthesis. 1994;03:245–6.
Takeuchi H. Raman structural markers of tryptophan and histidine side chains in proteins. Biopolymers. 2003;72:305–17.
Kerwin BA. Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: structure and degradation pathways. J Pharm Sci. 2007;98:3200–17.
Yao J, Dokuru DK, Noestheden M, Park SS, Kerwin BA, Jona J, et al. A quantitative kinetic study of polysorbate autooxidation: the role of unsaturated fatty acid ester substituents. Pharm Res. 2009;26:2303–13.
Fossey J, Lefort D, Sorba J. Free Radicals in Organic Chemistry. ed.: John Wiley & Sons; 1995. p 116.
Guo Q, Qian SY, Mason RP. Separation and identification of DMPO adducts of oxygen-centered radicals formed from organic hydroperoxides by HPLC-ESR, ESI-MS and MS/MS. J Am Soc Mass Spectr. 2003;14:862–71.
Acknowledgements
The authors would like to thank Carey Brennar and Dr. Scott Buckel for initially asking what the new peak was observed by SE HPLC and Drew Nichols for his initial work limiting some of the possibilities. We also would like to thank Dr. Songpon Deechongkit and Dr. David Hambly for many thoughtful discussions, Tsang-Lin Hwang for NMR analysis and Dr. Mike Treuheit for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mason, B.D., McCracken, M., Bures, E.J. et al. Oxidation of Free L-histidine by tert-Butylhydroperoxide. Pharm Res 27, 447–456 (2010). https://doi.org/10.1007/s11095-009-0032-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-009-0032-y