Abstract
Purpose
To investigate the local and global mobility in amorphous sucrose and trehalose and their potential implications on physical stability.
Methods
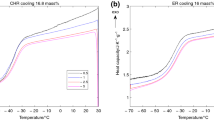
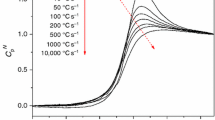
Amorphous sucrose was prepared by lyophilization while amorphous trehalose was prepared by dehydration of trehalose dihydrate. The variation in the effective activation energy of α-relaxation through glass transition has been determined by applying an isoconversional method. β-Relaxations were detected as shallow peaks, at temperatures below the glass transition temperature, caused by annealing glassy samples at different temperatures and subsequently heating at different rates in a differential scanning calorimeter. The effect of heating rate on the β-relaxation peak temperature formed the basis for the calculation of the activation energy.
Results
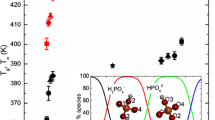
α-Relaxations in glassy trehalose were characterized by larger activation energy barrier compared to sucrose, attributable to a more compact molecular structure of trehalose. The effect of temperature on viscous flow was greater in trehalose which can have implications on lyophile collapse. The size of the cooperatively rearranging regions was about the same for sucrose and trehalose suggesting similar dynamic heterogeneity at their respective glass transition temperatures. The activation energy of β-relaxations increased with annealing temperature due to increasing cooperative motions and the increase was larger in sucrose. The temperature at which β-relaxation was detected for a given annealing time was much less in sucrose implying that progression of local motions to cooperative motions occurred at lower temperatures in sucrose.
Conclusions
Trehalose, having a lower free volume in the glassy state due to a more tightly packed molecular structure, is characterized by larger activation energies of α-relaxation and experiences a greater effect of temperature on the reduction in the activation energy barrier for viscous flow. The pronounced increase in cooperative motions in sucrose upon annealing at temperatures below (T g −50) suggest that even a small excursion in temperature could result in a significant increase in mobility.











Similar content being viewed by others
References
C. W. Pouton. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur. J. Pharm. Sci. 29:278–287 (2006). doi:10.1016/j.ejps.2006.04.016.
W. Wang. Instability, stabilization and formulation of liquid protein pharmaceuticals. Int. J. Pharm. 185:129–188 (1999). doi:10.1016/S0378-5173(99)00152-0.
L. Yu. Amorphous pharmaceutical solids: preparation, characterization and stabilization. Adv. Drug Deliv. Rev. 48:27–42 (2001). doi:10.1016/S0169-409X(01)00098-9.
B. C. Hancock, and G. Zografi. Characteristics and significance of the amorphous state in pharmaceutical systems. J. Pharm. Sci. 86:1–12 (1997). doi:10.1021/js9601896.
D. Q. M. Craig, P. G. Royall, V. L. Kett, and M. L. Hopton. The relevance of the amorphous state to pharmaceutical dosage forms: glassy drugs and freeze-dried systems. Int. J. Pharm. 179:179–207 (1999). doi:10.1016/S0378-5173(98)00338-X.
L. Kreilgaard, S. Frokjaer, J. M. Flink, T. W. Randolph, and J. F. Carpenter. Effects of additives on the stability of Humicola lanuginosa lipase during freeze-drying and storage in the dried solid. J. Pharm. Sci. 88:281–290 (1999). doi:10.1021/js980399d.
J. M. E. Sarciaux, and M. Hageman. Effects of bovine somatotropin (rbSt) concentration at different moisture levels on the physical stability of sucrose in freeze-dried rbSt/sucrose mixtures. J. Pharm. Sci. 86:365–371 (1997). doi:10.1021/js960217k.
K. Izutsu, S. Yoshioka, and S. Kojima. Physical stability and protein stability of freeze-dried cakes during storage at elevated temperatures. Pharm. Res. 11:995–999 (1994). doi:10.1023/A:1018931319772.
X. M. Zeng, G. P. Martin, and C. Marriott. Effects of molecular weight of polyvinylpyrrolidone on the glass transition and crystallization of co-lyophilized sucrose. Int. J. Pharm. 218:63–73 (2001). doi:10.1016/S0378-5173(01)00613-5.
S. Passot, F. Fonseca, M. Alarcon-lorca, D. Rolland, and M. Marin. Physical characterisation of formulations for the development of two stable freeze-dried proteins during both dried and liquid storage. Eur. J. Pharm. Biopharm. 60:335–348 (2005). doi:10.1016/j.ejpb.2005.02.013.
Y. H. Liao, M. B. Brown, T. Nazir, A. Quader, and G. P. Martin. Effects of sucrose and trehalose on the preservation of the native structure of spray-dried lysozyme. Pharm. Res. 19:1847–1853 (2002). doi:10.1023/A:1021445608807.
W. Q. Sun, and P. Davidson. Protein inactivation in amorphous sucrose and trehalose matrixes: effects of phase separation and crystallization. Biochim. Biophys. Acta. 1425:235–244 (1998).
S. L. Shamblin, X. Tang, L. Chang, B. C. Hancock, and M. J. Pikal. Characterization of the time scales of molecular motion in pharmaceutically important glasses. J. Phys. Chem. B. 103:4113–4121 (1999). doi:10.1021/jp983964+.
B. C. Hancock, and S. L. Shamblin. Molecular mobility of amorphous pharmaceuticals determined using differential scanning calorimetry. Thermochim. Acta. 380:95–107 (2001).
B. C. Hancock, S. L. Shamblin, and G. Zografi. Molecular mobility of amorphous pharmaceutical solids below their glass transition temperatures. Pharm. Res. 12:799–806 (1995). doi:10.1023/A:1016292416526.
I. Weuts, D. Kempen, K. Six, J. Peeters, G. Verreck, M. Brewster, and G. Van den Mooter. Evaluation of different calorimetric methods to determine the glass transition temperature and molecular mobility below Tg for amorphous drugs. Int. J. Pharm. 259:17–25 (2003). doi:10.1016/S0378-5173(03)00233-3.
G. P. Johari. Intrinsic mobility of molecular glasses. J. Chem. Phys. 58:1766–1770 (1973). doi:10.1063/1.1679421.
G. P. Johari, and M. Goldstein. Viscous liquids and the glass transition. II. Secondary relaxations in glasses of rigid molecules. J. Chem. Phys. 53:2372–2388 (1970).
K. L. Ngai. An extended coupling model description of the evolution of dynamics with time in supercooled liquids and ionic conductors. J. Phys.: Condensed Matter. 15:S1107–S1125 (2003). doi:10.1088/0953-8984/15/11/332.
T. Hikima, M. Hanaya, and M. Oguni. Microscopic observation of a peculiar crystallization in the glass transition region and β-process as potentially controlling the growth rate in triphenylethylene. J. Mol. Struct. 479:245–250 (1999). doi:10.1016/S0022-2860(98)00875-8.
J. Alie, J. Menegotto, P. Cardon, H. Duplaa, A. Caron, C. Lacabanne, and M. Bauer. Dielectric study of the molecular mobility and the isothermal crystallization kinetics of an amorphous pharmaceutical drug substance. J. Pharm. Sci. 93:218–233 (2004). doi:10.1002/jps.10520.
H. Fujimori, M. Mizukami, and M. Oguni. Calorimetric study of 1,3-diphenyl-1,1,3,3-tetramethyldisiloxane: emergence of α-, β-, and crystalline-glass transitions. J. Non-Crystal. Solids. 204:38–45 (1996). doi:10.1016/0022-3093(96)00177-9.
H. Fujimori, and M. Oguni. Calorimetric study of D,L-propene carbonate: observation of the β- as well α-glass transition in the supercooled liquid. J. Chem. Thermodyn. 26:367–378 (1994). doi:10.1006/jcht.1994.1046.
S. Vyazovkin, and I. Dranca. Physical stability and relaxation of amorphous indomethacin. J. Phys. Chem. B. 109:18637–18644 (2005). doi:10.1021/jp052985i.
S. Vyazovkin, and I. Dranca. Probing beta relaxation in pharmaceutically relevant glasses by using DSC. Pharm. Res. 23:422–428 (2006). doi:10.1007/s11095-005-9044-4.
H. S. Chen. On the mechanisms of structural relaxation in a Pd48Ni32P20 glass. J. Non-Crystal. Solids. 46:289–305 (1981). doi:10.1016/0022-3093(81)90007-7.
V. A. Bershtein, and V. M. Egorov. Differential Scanning Calorimetry of Polymers. Ellis Horwood, New York, 1994.
S. L. Shamblin, L. S. Taylor, and G. Zografi. Mixing behavior of colyophilized binary systems. J. Pharm. Sci. 87:694–701 (1998). doi:10.1021/JS9704801.
S. Vyazovkin. Evaluation of the activation energy of thermally stimulated solid-state reactions under an arbitrary variation of the temperature. J. Comput. Chem. 18:393–402 (1997). doi:10.1002/(SICI)1096-987X(199702)18:3<393::AID-JCC9>3.0.CO;2-P.
S. Vyazovkin. Modification of the integral isoconversional method to account for variation in the activation energy. J. Comput. Chem. 22:178–183 (2001). doi:10.1002/1096-987X(20010130)22:2<178::AID-JCC5>3.0.CO;2-#.
S. Vyazovkin, and N. Sbirazzouli. Isoconversional kinetic analysis of thermally stimulated processes in polymers. Macromol. Rapid Commun. 27:1515–1532 (2006). doi:10.1002/marc.200600404.
T. Ozawa. A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jap. 38:1881–1886 (1965). doi:10.1246/bcsj.38.1881.
H. J. Flynn, and L. A. Wall. General treatment of the thermogravimetry of polymers. J. Res. Natl. Bureau Standards. 70A:487–523 (1966).
S. Vyazovkin, N. Sbirazzouli, and I. Dranca. Variation in activation energy of the glass transition for polymers of different dynamic fragility. Macromol. Chem. Phys. 207:1126–1130 (2006). doi:10.1002/macp.200600095.
B. C. Hancock, C. R. Dalton, M. J. Pikal, and S. L. Shamblin. A pragmatic test of a simple calorimetric method for determining the fragility of some amorphous pharmaceutical materials. Pharm. Res. 15:762–767 (1998). doi:10.1023/A:1011931305755.
J. E. Green, R. Sitaula, A. Fowler, M. Toner, and S. Bhowmick. Enthalpic relaxation of convective desiccated trehalose–water glasses. Thermochim. Acta. 453:1–8 (2007). doi:10.1016/j.tca.2006.10.014.
C. Bhugra, S. Rambhatla, A. Bakri, S. P. Duddu, D. P. Miller, M. J. Pikal, and D. Lechuga-Ballesteros. Prediction of the onset of crystallization of amorphous sucrose below the calorimetric glass transition temperature from correlations with mobility. J. Pharm. Sci. 96:1258–1269 (2007). doi:10.1002/jps.20918.
J. C. Phillips. Ideally glassy hydrogen-bonded networks. Phys. Rev. B: Condensed Matter. 73:024210 (2006).
L. M. Wang, V. Velikov, and C. A. Angell. Direct determination of kinetic fragility indices of glassforming liquids by differential scanning calorimetry: kinetic versus thermodynamic fragilities. J. Chem. Phys. 117:10184–10192 (2002). doi:10.1063/1.1517607.
L. M. Wang, and C. A. Angell. Response to “Comment on ‘Direct determination of the fragility indices of glassforming liquids by differential scanning calorimetry: kinetic versus thermodynamic fragilities’” [J. Chem. Phys. 118, 10351.2003]. J. Chem. Phys. 118:10353–10355 (2003). doi:10.1063/1.1571815.
L. M. Wang, C. A. Angell, and R. Richert. Fragility and thermodynamics in nonpolymeric glass-forming liquids. J. Chem. Phys. 125:074505 (2006).
T. Higashiyama. Novel functions and applications of trehalose. Pure Appl. Chem. 74:1263–1269 (2002). doi:10.1351/pac200274071263.
G. Adam, and J. H. Gibbs. On the temperature dependence of cooperative relaxation properties in glass-forming liquids. J. Chem. Phys. 43:139–146 (1965). doi:10.1063/1.1696442.
S. H. Glarum. Dielectric relaxation of isoamyl bromide. J. Chem. Phys. 33:639–643 (1960). doi:10.1063/1.1731229.
E. Donth. The Glass Transition: Relaxation Dynamics in Liquids and Disordered Materials. Springer, Berlin, 2001.
E. Hempel, G. Hempel, A. Hensel, C. Schick, and E. Donth. Characteristic length of dynamic glass transition near Tg for a wide assortment of glass-forming substances. J. Phys. Chem. B. 104:2460–2466 (2000). doi:10.1021/jp991153f.
S. Vyazovkin, and I. Dranca. Comparative relaxation dynamics of glucose and maltitol. Pharm. Res. 23:2158–2164 (2006). doi:10.1007/s11095-006-9050-1.
A. D. Gusseme, L. Carpentier, J. F. Willart, and M. Descamps. Molecular Mobility in supercooled trehalose. J. Phys. Chem. B. 107:10879–10886 (2003). doi:10.1021/jp0343234.
S. Vyazovkin, and I. Dranca. Activation energies derived from the pre-glass transition annealing peaks. Thermochim. Acta. 446:140–146 (2006). doi:10.1016/j.tca.2006.04.017.
A. Kudlik, S. Benkhof, T. Blochowicz, C. Tschirwitz, and E. Rossler. The dielectric response of simple organic glass formers. J. Mol. Struct. 479:201–218 (1999). doi:10.1016/S0022-2860(98)00871-0.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dranca, I., Bhattacharya, S., Vyazovkin, S. et al. Implications of Global and Local Mobility in Amorphous Sucrose and Trehalose as Determined by Differential Scanning Calorimetry. Pharm Res 26, 1064–1072 (2009). https://doi.org/10.1007/s11095-008-9817-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9817-7