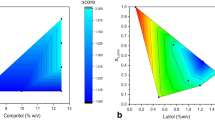
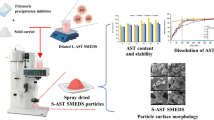
Abstract
Purpose
Drying of nanosuspensions can cause destabilization of the particles, leading to irreversible aggregation. In order to prepare an effective solid dosage form for a nanosuspension, it is imperative that the spray-dried nanoparticles should go back to their original particle size when reconstituted in an aqueous system. This case study examines impact of various formulation and processing parameters on redispersibility of the spray dried nanoparticles.
Methods
Nanosuspensions were prepared using the microprecipitation–homogenization process. Spray drying of nanosuspensions was achieved using a lab-scale Buchi spray dryer.
Results
Formulation components appeared to have the most significant impact on redispersibility of spray dried particles. Absence of surface charge led to particles that could not be redispersed. On the other hand, charged particles stabilized with an appropriate sugar led to spray dried powders that were flowable and easily redispersible. Dissolution testing showed the presence of two phases—a lag phase that represented dispersion of the loose aggregates, and dissolution of the dispersed nanoparticles.
Conclusions
Nanosuspensions of a poorly soluble drug could be spray dried to obtain flowable powders that could be easily redispersed. These optimized powders also showed significantly improved dissolution rates as compared to the micronized drug, or unoptimized nanosuspensions.




Similar content being viewed by others
References
K. R. Horspool, and C. A. Lipinski. Advancing new drug delivery concepts to gain the lead. Drug Deliv. Technol. 3:34–46 (2003).
M. Hariharan, L. D. Ganorkar, G. E. Amidon, E. Cavallo, P. Gatti, M. Hageman, I. Choo, J. L. Miller, and M. Shah. Reducing the time to develop and manufacture formulations for first oral dose in humans. Pharm. Tech. 27:68–84 (2003).
M. V. Chaubal. Application of drug delivery technologies in lead candidate selection and optimization. Drug Discov. Today. 9(14):603–609 (2004).
J. E. Kipp. The role of solid nanoparticle technology in the parenteral delivery of poorly water-soluble drugs. Int. J. Pharm. 284(1–2):109–22 (2004).
C. Schmidt, and R. Bodmeier. Incorporation of polymeric nanoparticles into solid dosage forms. J. Controlled Release. 57:115–125 (1999).
W. Abdelwahed, G. Degobert, S. Stainmesse, and H. Fessi. Freeze-drying of nanoparticles: formulation, process and storage considerations. Adv. Drug. Deliv. Rev. 58(15):1688–713 (2006).
S. Hirsjarvi, L. Peltonen, L. Kainu, and J. Hirvonen. Freeze-drying of low molecular weight poly(l-lactic acid) nanoparticles: effect of cryo- and lyoprotectants. J. Nanosci. Nanotechnol. 6(9–10):3110–3117 (2006).
A. M. Layre, P. Couvreur, J. Richard, D. Requier, N. Eddine Ghermani, and R. Gref. Freeze-drying of composite core-shell nanoparticles. Drug. Dev. Ind. Pharm. 32(7):839–846 (2006).
W. Abdelwahed, G. Degobert, and H. Fessi. Investigation of nanocapsules stabilization by amorphous excipients during freeze-drying and storage. Eur. J. Pharm. Biopharm. 63(2):87–94 (2006).
J. Lee. Drug nano- and microparticles processed into solid dosage forms: physical properties. J. Pharm. Sci. 92(10):2057–2068 (2003).
C. Freitas, and R. H. Muller. Spray drying of solid lipid nanoparticles (SLN TM). Eur. J. Pharm. Biopharm. 46(2):145–151 (1998).
S. X. Yin, M. Franchini, J. Chen, A. Hsieh, S. Jen, T. Lee, M. Hussain, and R. Smith. Bioavailability enhancement of a COX-2 inhibitor, BMS-347070, from a nanocrystalline dispersion prepared by spray drying. J. Pharm. Sci. 94(7):1598–1607 (2005).
R. Vehring. Pharmaceutical particle engineering via spray drying. Pharm. Res. 25(5):999–1022 (2008).
P. Johansen, H. P. Merkle, and B. Gander. Technological considerations related to the up-scaling of protein microencapsulation by spray-drying. Eur. J. Pharm. Biopharm. 50(3):413–417 (2000).
B. Rabinow, J. Kipp, P. Papadopoulos, J. Wong, J. Glosson, J. Gass, C. S. Sun, T. Wielgos, R. White, C. Cook, K. Barker, and K. Wood. Itraconazole IV nanosuspension enhances efficacy through altered pharmacokinetics in the rat. Int. J. Pharm. 339(1–2):251–260 (2007).
B. E. Rabinow. Nanosuspensions in drug delivery. Nat. Rev. Drug. Discov. 3(9):785–796 (2004).
M. T. Crisp, C. J. Tucker, T. L. Rogers, R. O. Williams 3rd, and K. P. Johnston. Turbidimetric measurement and prediction of dissolution rates of poorly soluble drug nanocrystals. J. Control. Release. 117:351–359 (2007).
S. N. Pace, G. W. Pace, I. Parikh, and A. K. Mishra. Novel injectable formulations of insoluble drugs. Pharm. Technol. 23:116–134 (1999).
F. Iskandar, L. Gradon, and K. Okuyama. Control of the morphology of nanostructured particles prepared by the spray drying of a nanoparticle sol. J. Colloid Interface Sci. 265(2):296–303 (2003).
D. Lide. CRC Handbook of Chemistry and Physics. 88th Edition. Informa, NY, 2007.
L. Kervinen, and J. Yliruusi. Modelling S-shaped dissolution curves. Int. J. Pharm. 92(1–3):115–122 (1993).
A. Dokoumetzidis, V. Papadopoulou, and P. Macheras. Analysis of dissolution data using modified versions of Noyes–Whitney equation and the Weibull function. Pharm Res. 23(2):256–261 (2006).
B. Van Eerdenbrugh, L. Froyen, J. A. Martens, N. Blaton, P. Augustijns, M. Brewster, and G. Van den Mooter. Characterization of physico-chemical properties and pharmaceutical performance of sucrose co-freeze-dried solid nanoparticulate powders of the anti-HIV agent loviride prepared by media milling. Int. J. Pharm. 338(1–2):198–206 (2007).
Acknowledgments
The authors would like to thank Sabine Graham, Baxter Healthcare for zeta potential measurements and Drs. Jane Werling and Sarah Lee for review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chaubal, M.V., Popescu, C. Conversion of Nanosuspensions into Dry Powders by Spray Drying: A Case Study. Pharm Res 25, 2302–2308 (2008). https://doi.org/10.1007/s11095-008-9625-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9625-0