Abstract
Purpose
Polysaccharides such as chondroitin play a potent role in tumor growth, tissue repair and angiogenesis. These properties make chondroitin a good candidate for novel drug delivery systems. Diammine dicarboxylic acid platinum (DDAP), a novel polymeric platinum compound, was developed by conjugating the platinum analogue to aspartate–chondroitin for drug delivery to tumor cells. DDAP improves platinum solubility which may reduce systemic toxicity and be more efficacious than cisplatin in killing tumor cells.
Methods
We tested and compared the cytotoxic effects of DDAP and CDDP on the platinum-sensitive 2008 and A2780 ovarian cancer cell lines and their platinum-resistant sublines 2008.C13 and A2780cis; we also investigated DDAP’s mechanism of action.
Results
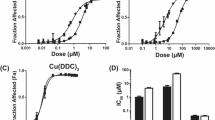
In the platinum-sensitive cell lines, the cytotoxic effects of DDAP and CDDP were comparable. However, in the platinum-resistant sublines, significantly greater cell-growth inhibition was induced by DDAP than by CDDP, especially at lower doses. DDAP also induced more apoptosis than CDDP did in the 2008.C13 subline, which was partially mediated by the caspase 3-dependent pathway. In addition, lower (but not higher) doses of DDAP arrested 90% of S-phase 2008.C13 cells, which might be associated with up-regulation of p21 and maintenance of low cyclin A expression. Furthermore, greater cellular uptake of DDAP was seen in platinum-resistant than in platinum-sensitive ovarian cancer cells.
Conclusions
Low-dose DDAP enhances drug delivery to platinum-resistant ovarian cancer cells and substantially inhibits their growth by inducting apoptosis and arresting cells in the S-phase, suggesting that DDAP may overcome platinum resistance in ovarian cancer.








Similar content being viewed by others
REFERENCES
A. Jemal, L. X. Clegg, E. Ward, L. A. Ries, X. Wu, P. M. Jamison, P. A. Wingo, H. L. Howe, R. N. Anderson, and B. K. Edwards. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 101:3–27 (2004).
J. J. Kavanagh, S. Pecoreli, A. Dipertrillo, and N. Einhorn. Chemotherapy in advanced ovarian cancer. Blackwell Scientific, Cambrige, MA, 1998.
W. P. McGuire, W. J. Hoskins, M. F. Brady, P. R. Kucera, E. E. Partridge, K. Y. Look, D. L. Clarke-Pearson, and M. Davidson. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer [see comments]. N Engl J Med. 334:1–6 (1996).
M. J. Piccart, H. Lamb, and J. B. Vermorken. Current and future potential roles of the platinum drugs in the treatment of ovarian cancer. Ann Oncol. 12:1195–1203 (2001).
J. T. Thigpen. Chemotherapy for advanced ovarian cancer: overview of randomized trials. Semin Oncol. 27:11–16 (2000).
G. Giaccone. Clinical perspectives on platinum resistance. Drugs. 59(Suppl 4):9–17 (2000)discussion 37–18.
S. Manic, L. Gatti, N. Carenini, G. Fumagalli, F. Zunino, and P. Perego. Mechanisms controlling sensitivity to platinum complexes: role of p53 and DNA mismatch repair. Curr Cancer Drug Targets. 3:21–29 (2003).
C. Balch, T. H. Huang, R. Brown, and K. P. Nephew. The epigenetics of ovarian cancer drug resistance and resensitization. Am J Obstet Gynecol. 191:1552–1572 (2004).
W. P. McGuire, and R. F. Ozols. Chemotherapy of advanced ovarian cancer. Semin Oncol. 25:340–348 (1998).
W. P. McGuire 3rd, and M. Markman. Primary ovarian cancer chemotherapy: current standards of care. Br J Cancer. 89(Suppl 3):S3–8 (2003).
M. A. Fuertes, J. Castilla, C. Alonso, and J. M. Perez. Novel concepts in the development of platinum antitumor drugs. Curr Med Chem Anti-Canc Agents. 2:539–551 (2002).
N. J. Moreland, M. Illand, Y. T. Kim, J. Paul, and R. Brown. Modulation of drug resistance mediated by loss of mismatch repair by the DNA polymerase inhibitor aphidicolin. Cancer Res. 59:2102–2106 (1999).
Z. H. Siddik. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 22:7265–7279 (2003).
F. M. Muggia, and G. Los. Platinum resistance: laboratory findings and clinical implications. Stem Cells. 11:182–193 (1993).
A. de Graeff, R. J. Slebos, and S. Rodenhuis. Resistance to cisplatin and analogues: mechanisms and potential clinical implications. Cancer Chemother Pharmacol. 22:325–332 (1988).
M. J. McKeage. New-generation platinum drugs in the treatment of cisplatin-resistant cancers. Expert Opin Investig Drugs. 14:1033–1046 (2005).
G. Biagini, A. Pugnaloni, A. Damadei, A. Bertani, A. Belligolli, V. Bicchiega, and R. Muzzarelli. Morphological study of the capsular organization around tissue expanders coated with N-carboxybutyl chitosan. Biomaterials. 12:287–291 (1991).
J. S. Pieper, P. B. van Wachem, M. J. A. van Luyn, L. A. Brouwer, T. Hafmans, J. H. Veerkamp, and T. H. van Kuppevelt. Attachment of glycosaminoglycans to collagenous matrices modulates the tissue response in rats. Biomaterials. 21:1689–1699 (2000).
M. Prabaharan, and J. F. Mano. Chitosan-based particles as controlled drug delivery systems. Drug Deliv. 12:41–57 (2005).
A. Polykratis, P. Katsoris, J. Courty, and E. Papadimitriou. Characterization of heparin affin regulatory peptide signaling in human endothelial cells. J Biol Chem. 280:22454–22461 (2005).
M. K. Chourasia, and S. K. Jain. Polysaccharides for colon targeted drug delivery. Drug Deliv. 11:129–148 (2004).
V. R. Sinha, and R. Kumria. Polysaccharides in colon-specific drug delivery. Int J Pharm. 224:19–38 (2001).
C. Haase, R. Bergmann, F. Fuechtner, A. Hoepping, and J. Pietzsch. l-type amino acid transporters LAT1 and LAT4 in cancer: uptake of 3-O-methyl-6-18F-fluoro-l-dopa in human adenocarcinoma and squamous cell carcinoma in vitro and in vivo. J Nucl Med. 48:2063–2071 (2007).
C. Li, J. E. Price, L. Milas, N. R. Hunter, S. Ke, D. F. Yu, C. Charnsangavej, and S. Wallace. Antitumor activity of poly(l-glutamic acid)-paclitaxel on syngeneic and xenografted tumors. Clin Cancer Res. 5:891–897 (1999).
Y. Sedletska, M. J. Giraud-Panis, and J. M. Malinge. Cisplatin is a DNA-damaging antitumour compound triggering multifactorial biochemical responses in cancer cells: importance of apoptotic pathways. Curr Med Chem Anticancer Agents. 5:251–265 (2005).
S. Fulda, M. Los, C. Friesen, and K.M. Debatin. Chemosensitivity of solid tumor cells in vitro is related to activation of the CD95 system. Int J Cancer. 76:105–114 (1998).
H. Kojima, K. Endo, H. Moriyama, Y. Tanaka, E. S. Alnemri, C. A. Slapak, B. Teicher, D. Kufe, and R. Datta. Abrogation of mitochondrial cytochrome c release and caspase-3 activation in acquired multidrug resistance. J Biol Chem. 273:16647–16650 (1998).
K. M. Henkels, and J. J. Turchi. Cisplatin-induced apoptosis proceeds by caspase-3-dependent and -independent pathways in cisplatin-resistant and -sensitive human ovarian cancer cell lines. Cancer Res. 59:3077–3083 (1999).
G. D. Diaz, Q. Li, and R. H. Dashwood. Caspase-8 and apoptosis-inducing factor mediate a cytochrome c-independent pathway of apoptosis in human colon cancer cells induced by the dietary phytochemical chlorophyllin. Cancer Res. 63:1254–1261 (2003).
B. S. Cummings, G. R. Kinsey, L. J. Bolchoz, and R. G. Schnellmann. Identification of caspase-independent apoptosis in epithelial and cancer cells. J Pharmacol Exp Ther. 310:126–134 (2004).
R. E. Aird, J. Cummings, A. A. Ritchie, M. Muir, R. E. Morris, H. Chen, P. J. Sadler, and D. I. Jodrell. In vitro and in vivo activity and cross resistance profiles of novel ruthenium (II) organometallic arene complexes in human ovarian cancer. Br J Cancer. 86:1652–1657 (2002).
V. V. Ogryzko, P. Wong, and B. H. Howard. WAF1 retards S-phase progression primarily by inhibition of cyclin-dependent kinases. Mol Cell Biol. 17:4877–4882 (1997).
V. Gottifredi, K. McKinney, M. V. Poyurovsky, and C. Prives. Decreased p21 levels are required for efficient restart of DNA synthesis after S phase block. J Biol Chem. 279:5802–5810 (2004).
A. L. Gartel, and A. L. Tyner. Transcriptional regulation of the p21((WAF1/CIP1)) gene. Exp Cell Res. 246:280–289 (1999).
S. Gilfillan, E. L. Ho, M. Cella, W. M. Yokoyama, and M. Colonna. NKG2D recruits two distinct adapters to trigger NK cell activation and costimulation. Nat Immunol. 3:1150–1155 (2002).
Acknowledgment
We thank Tamara Locke in the Department of Scientific Publications at M. D. Anderson Cancer Center for editing this manuscript, as well as Ling Chen and Richard Mendez for their assistance with the experiments. This work was supported by M. D. Anderson’s institutional grant and Core Grant (NCI grant 5P30CA016672-32).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zheng, H., Hu, W., Yu, D. et al. Diammine Dicarboxylic Acid Platinum Enhances Cytotoxicity in Platinum-Resistant Ovarian Cancer Cells through Induction of Apoptosis and S-Phase Cell Arrest. Pharm Res 25, 2272–2282 (2008). https://doi.org/10.1007/s11095-008-9621-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9621-4