Abstract
Purpose
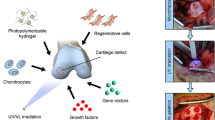
Synthetic hydrogels fabricated from photopolymerization are attractive for tissue engineering for their controlled macroscopic properties, the ability to incorporate biological functionalities, and cell encapsulation. The goal of the present study was to exploit the attractive features of synthetic hydrogels to elucidate the role of gel structure and chemistry in regulating biomechanical cues.
Methods
Cartilage cells were encapsulated in poly(ethylene glycol) (PEG) hydrogels with different crosslinking densities. Cellular deformation was examined as a function of gel crosslinking. The effects of continuous versus intermittent dynamic loading regimens were examined. RGD, a cell adhesion peptide, was incorporated into PEG gels and subjected to mechanical loading. Chondrocyte morphology and activity was assessed by anabolic and catabolic ECM gene expression and matrix production by collagen and glycosaminoglycan production.
Results
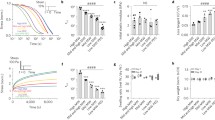
Cell deformation was mediated by gel crosslinking. In the absence of loading, anabolic activity was moderately upregulated while catabolic activity was significantly inhibited regardless of gel crosslinking. Dynamic loading enhanced anabolic activities, but continuous loading inhibited catabolic activity, while intermittent loading stimulated catabolic activity. RGD acted as a mechanoreceptor to influence tissue deposition.
Conclusions
We demonstrate the ability to regulate biomechanical cues through manipulations in the gel structure and chemistry and cartilage tissue engineering.




Similar content being viewed by others
References
S. J. Bryant, and K. S. Anseth. Hydrogel properties influence ecm production by chondrocyte photoencapsulated in poly(ethylene glycol) hydrogels. J. Biomed. Mater. Res. 59:63–72 (2002).
S. J. Bryant, C. R. Nuttelman, and K. S. Anseth. Cytocompatibility of ultraviolet and visible light photoinitiating systems on cultured nih/3t3 fibroblasts in vitro. J. Biomater. Sci. Polym. Ed. 11:439–457 (2000).
J. Elisseeff, K. Anseth, D. Sims, W. McIntosh, M. Randolph, and R. Langer. Transdermal photopolymerization for minimally invasive implantation. Proc. Natl. Acad. Sci. U. S. A. 96:3104–3107 (1999).
J. A. Burdick, M. N. Mason, A. D. Hinman, K. Thorne, and K. S. Anseth. Delivery of osteoinductive growth factors from degradable peg hydrogels influences osteoblast differentiation and mineralization. J. Control. Release. 83:53–63 (2002).
A. S. Sawhney, C. P. Pathak, and J. A. Hubbell. Bioerodible hydrogels based on photopolymerized poly(ethylene glycol)-co-poly(alpha-hydroxy acid) diacrylate macromers. Macromolecules. 26:581–587 (1993).
A. T. Metters, K. S. Anseth, and C. N. Bowman. Fundamental studies of a novel, biodegradable peg-b-pla hydrogel. Polymer. 41:3993–4004 (2000).
K. A. Smeds, and M. W. Grinstaff. Photocrosslinkable polysaccharides for in situ hydrogel formation. J. Biomed. Mater. Res. 54:115–121 (2001).
P. Martens, and K. S. Anseth. Characterization of hydrogels formed from acrylate modified poly(vinyl alcohol) macromers. Polymer. 41:7715–7722 (2000).
J. Baier Leach, K. A. Bivens, C. W. Patrick, and C. E. Schmidt. Photocrosslinked hyaluronic acid hydrogels: Natural, biodegradable tissue engineering scaffolds. Biotechnol. Bioeng. 82:578–589 (2003).
S. J. Bryant, K. A. Davis-Areharet, N. Luo, R. K. Shoemaker, J. A. Arthur, and K. S. Anseth. Synthesis and characterization of photopolymerized multifunctional hydrogels: Water-soluble poly(vinyl alcohol) and chondroitin sulfate macromers for chondrocyte encapsulation. Macromolecules. 37:6726–6733 (2004).
D. A. Wang, C. G. Williams, Q. A. Li, B. Sharma, and J. H. Elisseeff. Synthesis and characterization of a novel degradable phosphate-containing hydrogel. Biomaterials. 24:3969–3980 (2003).
Q. Li, C. G. Williams, D. D. N. Sun, J. Wang, K. Leong, and J. H. Elisseeff. Photocrosslinkable polysaccharides based on chondroitin sulfate. J. Biomed. Mater. Res. A. 68A:28–33 (2004).
Y. Yeo, J. A. Burdick, C. B. Highley, R. Marini, R. Langer, and D. S. Kohane. Peritoneal application of chitosan and uv-cross-linkable chitosan. J. Biomed. Mater. Res. A. 78A:668–675 (2006).
S. H. M. Sontjens, D. L. Nettles, M. A. Carnahan, L. A. Setton, and M. W. Grinstaff. Biodendrimer-based hydrogel scaffolds for cartilage tissue repair. Biomacromolecules. 7:310–316 (2006).
J. Elisseeff, W. McIntosh, K. Anseth, S. Riley, P. Ragan, and R. Langer. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J. Biomed. Mater. Res. 51:164–171 (2000).
N. S. Hwang, S. Varghese, Z. Zhang, and J. Elisseeff. Chondrogenic differentiation of human embryonic stem cell-derived cells in arginine-glycine-aspartate modified hydrogels. Tissue Eng. 12:2695–2706 (2006).
S. J. Bryant, K. L. Durand, and K. S. Anseth. Manipulations in hydrogel chemistry control photoencapsulated chondrocyte behavior and their extracellular matrix production. J. Biomed. Mater. Res. A. 67A:1430–1436 (2003).
M. Dadsetan, J. P. Szatkowski, M. J. Yaszemski, and L. C. Lu. Characterization of photo-cross-linked oligo[poly(ethylene glycol) fumarate] hydrogels for cartilage tissue engineering. Biomacromolecules. 8:1702–1709 (2007).
J. A. Burdick, and K. S. Anseth. Photoencapsulation of osteoblasts in injectable rgd-modified peg hydrogels for bone tissue engineering. Biomaterials. 23:4315–4323 (2002).
D. S. W. Benoit, A. R. Durney, and K. S. Anseth. Manipulations in hydrogel degradation behavior enhance osteoblast function and mineralized tissue formation. Tissue Eng. 12:1663–1673 (2006).
D. A. Wang, C. G. Williams, F. Yang, N. Cher, H. Lee, and J. H. Elisseeff. Bioresponsive phosphoester hydrogels for bone tissue engineering. Tissue Eng. 11:201–213 (2005).
M. J. Mahoney, and K. S. Anseth. Three-dimensional growth and function of neural tissue in degradable polyethylene glycol hydrogels. Biomaterials. 27:2265–2274 (2006).
V. A. Liu, and S. N. Bhatia. Three-dimensional photopatterning of hydrogels containing living cells. Biomed. Microdevices. 4:257–266 (2002).
S. Gerecht, J. A. Burdick, L. S. Ferreira, S. A. Townsend, R. Langer, and G. Vunjak-Novakovic. Hyaluronic acid hydrogen for controlled self-renewal and differentiation of human embryonic stem cells. Proc. Natl. Acad. Sci. U. S. A. 104:11298–11303 (2007).
G. M. Cruise, D. S. Scharp, and J. A. Hubbell. Characterization of permeability and network structure of interfacially photopolymerized poly(ethylene glycol) diacrylate hydrogels. Biomaterials. 19:1287–94 (1998).
N. A. Peppas, J. Z. Hilt, A. Khademhosseini, and R. Langer. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 18:1345–1360 (2006).
M. N. Mason, A. T. Metters, C. N. Bowman, and K. S. Anseth. Predicting controlled-release behavior of degradable pla-b-peg-b-pla hydrogels. Macromolecules. 34:4630–4635 (2001).
M. M. Knight, D. A. Lee, and D. L. Bader. Distribution of chondrocyte deformation in compressed agarose gel using confocal microscopy. Cell. Eng. 1:97–102 (1996).
P. M. Freeman, R. N. Natarajan, J. H. Kimura, and T. P. Andriacchi. Chondrocyte cells respond mechanically to compressive loads. J. Orthop. Res. 12:311–320 (1994).
I. Villanueva, D. S. Hauschulz, D. Mejic, and S. J. Bryant. Static and dynamic compressive strains influence nitric oxide production and chondrocyte bioactivity when encapsulated in peg hydrogels of different crosslinking densities. Osteoarthr. Cartil. in press (2008).
G. D. Nicodemus, I. Villanueva, and S. J. Bryant. Mechanical stimulation of tmj condylar chondrocytes encapsulated in peg hydrogels. J. Biomed. Mater. Res. A. 83A:323–331 (2007).
P. Chomczynski. A reagent for the single-step simultaneous isolation of rna, dna and proteins from cell and tissue samples. Biotechniques. 15:532–& (1993).
L. Galois, S. Hutasse, D. Cortial, C. F. Rousseau, L. Grossin, M. C. Ronziere, D. Herbage, and A. M. Freyria. Bovine chondrocyte behaviour in three-dimensional type i collagen gel in terms of gel contraction, proliferation and gene expression. Biomaterials. 27:79–90 (2006).
A. P. Hollander, T. F. Heathfield, C. Webber, Y. Iwata, R. Bourne, C. Rorabeck, and A. P. Poole. Increased damage to type-ii collagen in osteoarthritic articular-cartilage detected by a new immunoassay. J. Clin. Invest. 93:1722–1732 (1994).
R. W. Farndale, D. J. Buttle, and A. J. Barrett. Improved quantitation and discrimination of sulfated glycosaminoglycans by use of dimethylmethylene blue. Biochim. Biophys. Acta. 883:173–177 (1986).
Y. J. Kim, R. L. Y. Sah, J. Y. H. Doong, and A. J. Grodzinsky. Fluorometric assay of DNA in cartilage explants using hoechst-33258. Anal. Biochem. 174:168–176 (1988).
F. Guilak, W. R. Jones, H. P. Ting-Beall, and G. M. Lee. The deformation behavior and mechanical properties of chondrocytes in articular cartilage. Osteoarthr. Cartil. 7:59–70 (1999).
M. M. Knight, J. M. Ross, A. F. Sherwin, D. A. Lee, D. L. Bader, and C. A. Poole. Chondrocyte deformation within mechanically and enzymatically extracted chondrons compressed in agarose. Biochim. Biophys. Acta. 1526:141–146 (2001).
F. Guilak, L. G. Alexopoulos, M. L. Upton, I. Youn, J. B. Choi, L. Cao, L. A. Setton, and M. A. Haider. The pericellular matrix as a transducer of biomechanical and biochemical signals in articular cartilage. Ann. N. Y. Acad. Sci. 1068:498–512 (2006).
R. L. Mauck, M. A. Soltz, C. C. B. Wang, D. D. Wong, P. H. G. Chao, W. B. Valhmu, C. T. Hung, and G. A. Ateshian. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J. Biomech. Eng. 122:252–260 (2000).
R. L. Mauck, B. A. Byers, X. Yuan, and R. S. Tuan. Regulation of cartilaginous ecm gene transcription by chondrocytes and mscs in 3d culture in response to dynamic loading. Biomech. Model. Mechanobiol. 6:113–125 (2007).
J. B. Fitzgerald, M. Jin, D. Dean, D. J. Wood, M. H. Zheng, and A. J. Grodzinsky. Mechanical compression of cartilage explants induces multiple time-dependent gene expression patterns and involves intracellular calcium and cyclic amp. J. Biol. Chem. 279:19502–19511 (2004).
J. N. A. De Croos, S. S. Dhaliwal, M. D. Grynpas, R. M. Pilliar, and R. A. Kandel. Cyclic compressive mechanical stimulation induces sequential catabolic and anabolic gene changes in chondrocytes resulting in increased extracellular matrix accumulation. Matrix Biology. 25:323–331 (2006).
G. Sharma, R. K. Saxena, and P. Mishra. Differential effects of cyclic and static pressure on biochemical and morphological properties of chondrocytes from articular cartilage. Clin. Biomech. 22:248–255 (2007).
C. A. Poole. Articular cartilage chondrons: Form, function and failure. J. Anat. 191:1–13 (1997).
E. Ruoslahti. Rgd and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 12:697–715 (1996).
M. Enomoto-Iwamoto, M. Iwamoto, K. Nakashima, Y. Mukudai, D. Boettiger, M. Pacifici, K. Kurisu, and F. Suzuki. Involvement of alpha 5 beta 1 integrin in matrix interactions and proliferation of chondrocytes. J. Bone Miner. Res. 12:1124–1132 (1997).
C. N. Salinas, B. B. Cole, A. M. Kasko, and K. S. Anseth. Chondrogenic differentiation potential of human mesenchymal stem cells photoencapsulated within poly(ethylene glycol)-arginine-glycine-aspartic acid-serine thiol-methacrylate mixed-mode networks. Tissue Eng. 13:1025–1034 (2007).
Acknowledgements
The authors acknowledge financial support from the NIH with a research grant from the NIDCR (K22 DE016608). The authors also acknowledge support from the Department of Education’s Graduate Assistantships in Areas of National Need Fellowship to GDN and IV and a NASA Harriet Jenkins Predoctoral Fellowship to IV.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bryant, S.J., Nicodemus, G.D. & Villanueva, I. Designing 3D Photopolymer Hydrogels to Regulate Biomechanical Cues and Tissue Growth for Cartilage Tissue Engineering. Pharm Res 25, 2379–2386 (2008). https://doi.org/10.1007/s11095-008-9619-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9619-y