Abstract
Purpose
The lithium disposition to cerebrospinal fluid (CSF) was evaluated in rats with acute renal failure (ARF) to examine whether electrolyte homeostasis of the CSF is perturbed by kidney dysfunction. In addition, the effects of renal failure on choroid plexial expressions of the Na+–K+–2Cl− co-transporter (NKCC1) and Na+/H+ exchanger (NHE1) were also studied.
Methods
After lithium was intravenously administered at a dose of 4 mmol/kg, its concentration profile in plasma was evaluated by collecting plasma specimens, while that in CSF was monitored with a microdialysis probe in the lateral ventricles. NKCC1 and NHE1 expressions were measured via the Western immunoblot method using membrane specimens prepared from the choroid plexus in normal and ARF rats.
Results
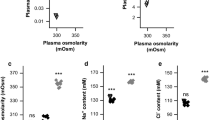
The lithium concentration in CSF of ARF rats was 30% lower than that of normal rats, while their plasma lithium profiles were almost the same, indicating that the lithium disposition to CSF was decreased in ARF rats. It was revealed that the choroid plexial expression of NKCC1 was increased by 40% in ARF rats, but that of NHE1 was unchanged.
Conclusion
ARF decreases the lithium disposition to CSF, possibly by promoting lithium efflux from CSF due to increased NKCC1 expression in the choroid plexus.





Similar content being viewed by others
References
J. Schnermann. Sodium transport deficiency and sodium balance in gene-targeted mice. Acta. Physiol. Scand. 173:59–66 (2001).
D. Biemesderfer, J. Pizzonia, A. Abu-Alfa, M. Exner, R. Reilly, P. Igarashi, and P. S. Aronson. NHE3: a Na+/H+ exchanger isoform of renal brush border. Am. J. Physiol. 265:F736–F742 (1993).
M. R. Kaplan, D. B. Mount, and E. Delpire. Molecular mechanisms of NaCl cotransport. Annu. Rev. Physiol. 58:649–668 (1996).
H. Garty, and L. G. Palmer. Epithelial sodium channels: function, structure, and regulation. Physiol. Rev. 77:359–396 (1997).
F. A. Leblond, L. Giroux, J. P. Villeneuve, and V. Pichette. Decreased in vivo metabolism of drugs in chronic renal failure. Drug Metab. Dispos. 28:1317–1320 (2000).
Y. J. Moon, A. K. Lee, H. C. Chung, E. J. Kim, S. H. Kim, D. C. Lee, I. Lee, S. G. Kim, and M. G. Lee. Effects of acute renal failure on the pharmacokinetics of chlorzoxazone in rats. Drug Metab. Dispos. 31:776–784 (2003).
T. Kimura, A. Kobayashi, M. Kobayashi, K. Numata, Y. Kawai, Y. Kurosaki, T. Nakayama, M. Mori, and M. Awai. Intestinal absorption of drugs in rats with glycerol-induced acute renal failure. Chem. Pharm. Bull. 36:1847–1856 (1988).
H. Tanabe, S. Taira, M. Taguchi, and Y. Hashimoto. Pharmacokinetics and hepatic extraction of metoprolol in rats with glycerol-induced acute renal failure. Biol. Pharm. Bull. 30:552–555 (2007).
F. A. Leblond, M. Petrucci, P. Dubé, G. Bernier, A. Bonnardeaux, and V. Pichette. Downregulation of intestinal cytochrome p450 in chronic renal failure. J. Am. Soc. Nephrol. 13:1579–1585 (2002).
H. Sun, L. Frassetto, and L. Z. Benet. Effects of renal failure on drug transport and metabolism. Pharmacol. Ther. 109:1–11 (2006).
Y. Masubuchi, M. Kawasaki, and T. Horie. Down-regulation of hepatic cytochrome P450 enzymes associated with cisplatin-induced acute renal failure in male rats. Arch. Toxicol. 80:347–353 (2006).
J. Naud, J. Michaud, C. Boisvert, K. Desbiens, F. A. Leblond, A. Mitchell, C. Jones, A. Bonnardeaux, and V. Pichette. Down-regulation of intestinal drug transporters in chronic renal failure in rats. J. Pharmacol. Exp. Ther. 320:978–985 (2007).
J. Dingemanse, M. Polhuijs, and M. Danhof. Altered pharmacokinetic- pharmacodynamic relationship of heptabarbital in experimental renal failure in rats. J. Pharmacol. Exp. Ther. 246:371–376 (1988).
M. Yasuhara, and G. Levy. Kinetics of drug action in disease states. XXVII. Effect of experimental renal failure on the pharmacodynamics of zoxazolamine and chlorzoxazone. J. Pharmacol. Exp. Ther. 246:165–169 (1988).
J. Kawakami, K. Ohashi, K. Yamamoto, Y. Sawada, and T. Iga. Effect of acute renal failure on neurotoxicity of enoxacin in rats. Biol. Pharm. Bull. 20:931–934 (1997).
Y. Ishiwata, K. Son, Y. Itoga, and M. Yasuhara. Effects of acute renal failure and ganciclovir on the pharmacodynamics of levofloxacin-induced seizures in rats. Biol. Pharm. Bull. 30:745–750 (2007).
M. Nagata, T. Fujichika, and M. Yasuhara. Effect of experimental renal failure and hypotonic hyponatremia on the pharmacodynamics of cefazolin-induced seizures in rats. Pharm. Res. 20:937–942 (2003).
B. S. Huang, W. J. Cheung, H. Wang, J. Tan, R. A. White, and F. H. Leenen. Activation of brain renin–angiotensin–aldosterone system by central sodium in Wistar rats. Am. J. Physiol. Heart Circ. Physiol. 291:H1109–H1117 (2006).
D. Mouginot, S. Laforest, and G. Drolet. Challenged sodium balance and expression of angiotensin type 1A receptor mRNA in the hypothalamus of Wistar and Dahl rat strains. Regul. Pept. 142:44–51 (2007).
E. Watanabe, T. Y. Hiyama, H. Shimizu, R. Kodama, N. Hayashi, S. Miyata, Y. Yanagawa, K. Obata, and M. Noda. Sodium-level-sensitive sodium channel Nax is expressed in glial laminate processes in the sensory circumventricular organs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290:R568–R576 (2006).
S. N. Orlov, and A. A. Mongin. Salt-sensing mechanisms in blood pressure regulation and hypertension. Am. J. Physiol. Heart Circ. Physiol. 293:H2039–H2053 (2007).
C. S. Chaurasia. In vivo microdialysis sampling: theory and applications. Biomed. Chromatogr. 13:317–332 (1999).
Y. Kurosaki, M. Tagawa, A. Omoto, H. Suito, Y. Komori, H. Kawasaki, and T. Aiba. Evaluation of intramuscular lateral distribution profile of topically administered acetaminophen in rats. Int. J. Pharm. 343:190–195 (2007).
S. Busch, B. C. Burckhardt, and W. Siffert. Expression of the human sodium/proton exchanger NHE-1 in Xenopus laevis oocytes enhances sodium/proton exchange activity and establishes sodium/lithium countertransport. Pflügers Arch. 429:859–869 (1995).
R. T. Timmer, and J. M. Sands. Lithium intoxication. J. Am. Soc. Nephrol. 10:666–674 (1999).
P. D. Brown, S. L. Davies, T. Speake, and I. D. Millar. Molecular mechanisms of cerebrospinal fluid production. Neuroscience. 129:957–970 (2004).
M. D. Plotkin, M. R. Kaplan, L. N. Peterson, S. R. Gullans, S. C. Hebert, and E. Delpire. Expression of the Na+–K+–2Cl− cotransporter BSC2 in the nervous system. Am. J. Physiol. 272:C173–C183 (1997).
Q. Wu, E. Delpire, S. C. Hebert, and K. Strange. Functional demonstration of Na+–K+–2Cl− cotransporter activity in isolated, polarized choroid plexus cells. Am. J. Physiol. 275:C1565–H1572 (1998).
N. H. Nakamura, D. R. Rosell, K. T. Akama, and B. S. McEwen. Estrogen and ovariectomy regulate mRNA and protein of glutamic acid decarboxylases and cation–chloride cotransporters in the adult rat hippocampus. Neuroendocrinology. 80:308–323 (2004).
Z. B. Redzic, J. E. Preston, J. A. Duncan, A. Chodobski, and J. Szmydynger-Chodobska. The choroid plexus–cerebrospinal fluid system: from development to aging. Curr. Top Dev. Biol. 71:1–52 (2005).
J. Praetorius. Water and solute secretion by the choroid plexus. Pflügers Arch. 454:1–18 (2007).
T. Aiba, M. Horiuchi, T. Makita, Y. Komori, H. Kawasaki, and Y. Kurosaki. Peritoneal dialysis alters tolbutamide pharmacokinetics in rats with experimental acute renal failure. Drug Metab. Pharmacokinet. 21:291–296 (2006).
G. Paxinos, and C. Watson. The Rat Brain in Stereotaxic Coordinates (2/e). Academic, New York, 1986.
R. D. Egleton, C. C. Campos, J. D. Huber, R. C. Brown, and T. P. Davis. Differential effects of diabetes on rat choroid plexus ion transporter expression. Diabetes. 52:1496–1501 (2003).
T. Aiba, Y. Takehara, M. Okuno, and Y. Hashimoto. Poor correlation between intestinal and hepatic metabolic rates of CYP3A4 substrates in rats. Pharm. Res. 20:745–748 (2003).
T. Aiba, M. Yoshinaga, K. Ishida, Y. Takehara, and Y. Hashimoto. Intestinal expression and metabolic activity of the CYP3A subfamily in female rats. Biol. Pharm. Bull. 28:311–315 (2005).
V. A. Murphy, and C. E. Johanson. Na+–H+ exchange in choroid plexus and CSF in acute metabolic acidosis or alkalosis. Am. J. Physiol. 258:F1528–F1537 (1990).
B. S. Huang, and F. H. Leenen. Brain amiloride-sensitive Phe-Met-Arg-Phe-NH2-gated Na+ channels and Na+-induced sympathoexcitation and hypertension. Hypertension. 39:557–561 (2002).
B. S. Huang, B. N. Van Vliet, and F. H. Leenen. Increases in CSF [Na+] precede the increases in blood pressure in Dahl S rats and SHR on a high-salt diet. Am. J. Physiol. Heart Circ. Physiol. 287:H1160–H1166 (2004).
T. S. Perrot-Sinal, C. J. Sinal, J. C. Reader, D. B. Speert, and M. M. McCarthy. Sex differences in the chloride cotransporters, NKCC1 and KCC2, in the developing hypothalamus. J. Neuroendocrinol. 19:302–308 (2007).
L. Ji, S. Masuda, H. Saito, and K. Inui. Down-regulation of rat organic cation transporter rOCT2 by 5/6 nephrectomy. Kidney Int. 62:514–524 (2002).
P. D. Metcalfe, and K. K. Meldrum. Sex differences and the role of sex steroids in renal injury. J. Urol. 176:15–21 (2006).
S. E. Mulroney, C. Woda, M. Johnson, and C. Pesce. Gender differences in renal growth and function after uninephrectomy in adult rats. Kidney Int. 56:944–953 (1999).
K. M. Park, J. I. Kim, Y. Ahn, A. J. Bonventre, and J. V. Bonventre. Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. J. Biol. Chem. 279:52282–52292 (2004).
K. Nakajima, H. Miyazaki, N. Niisato, and Y. Marunaka. Essential role of NKCC1 in NGF-induced neurite outgrowth. Biochem. Biophys. Res. Commun. 359:604–610 (2007).
H. Friess, Z. W. Zhu, F. F. di Mola, C. Kulli, H. U. Graber, A. Andren-Sandberg, A. Zimmermann, M. Korc, M. Reinshagen, and M. W. Büchler. Nerve growth factor and its high-affinity receptor in chronic pancreatitis. Ann. Surg. 230:615–624 (1999).
K. Bielefeldt, N. Ozaki, and G. F. Gebhart. Role of nerve growth factor in modulation of gastric afferent neurons in the rat. Am. Physiol. Gastrointest. Liver Physiol. 284:G499–G507 (2003).
N. E. Kremer, G. D’Arcangelo, S. M. Thomas, M. DeMarco, J. S. Brugge, and S. Halegoua. Signal transduction by nerve growth factor and fibroblast growth factor in PC12 cells requires a sequence of Src and Ras actions. J. Cell Biol. 115:809–819 (1991).
T. Ito, T. Suzuki, and H. Ichinose. Nerve growth factor-induced expression of the GTP cyclohydrolase I gene via Ras/MEK pathway in PC12D cells. J. Neurochem. 95:563–569 (2005).
M. D. Okusa, and L. J. Crystal. Clinical manifestations and management of acute lithium intoxication. Am. J. Med. 97:383–389 (1994).
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakae, R., Ishikawa, A., Niso, T. et al. Decreased Lithium Disposition to Cerebrospinal Fluid in Rats with Glycerol-induced Acute Renal Failure. Pharm Res 25, 2243–2249 (2008). https://doi.org/10.1007/s11095-008-9612-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9612-5