Abstract
Purpose
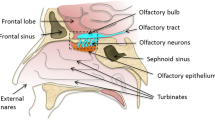
The aim of this study was to investigate and quantify drug movement to the brain via the neuro-olfactory system after intranasal dosing of four model drugs; three glycine receptor antagonists and one angiotensin antagonist.
Methods
The drugs were dosed to rats via intranasal or intravenous administration, after which a quantitative method for tissue distribution was utilised to determine drug distribution to the olfactory lobes, brain sections and the blood over 30 min. Autoradiography was used to visualise and quantify drug distribution throughout the brain and in the CSF. Micro-autoradiography was used to examine drug distribution throughout the olfactory nerve apparatus.
Results
The three glycine receptor antagonist compounds were transported to the CNS to differing degrees although they had similar molecular structures and similar physicochemical characteristics. All three compounds were shown to exploit a direct route of transport from nose to brain with Direct Transport Percentages (DTP) of 99.99%, 96.71% and 51.95%, respectively, although for the last molecule a major part of the brain content originated from systemic transport across the BBB. Intranasal administration of GR138950 resulted in over 3.5 times more drug in the olfactory lobes at 1 min post-dose compared to intravenous administration; and 5 times more drug was delivered to the olfactory lobes over 30 min. Micro-autoradiography showed that GR138950 could be found throughout the olfactory nerve apparatus. Autoradiography illustrated drug distribution throughout the brain and CSF, with drug concentrations in the CSF being equal or higher than in the brain tissue. It was determined that approximately 0.8% of the administered dose moved into the brain and CSF via the olfactory pathway over 30 min.
Conclusions
Intranasal administration resulted in greater delivery of the model drugs to the olfactory lobes and brain as compared to intravenous dosing. It is proposed that the drug moved through the neuro-olfactory system, primarily via paracellular pathways.






Similar content being viewed by others
References
W. M. Pardridge. Non-invasive drug delivery to the human brain using endogenous blood-brain barrier transport systems. Pharm. Sci. Technol. To. 2:49–59 (1999).
P. A. Hilger. Applied anatomy and physiology of the nose. In: Otolaryngology. A textbook of ear, nose and throat diseases. W. B. Saunders, Philadelphia, pp177–195 (1989).
L. Illum. Is nose-to-brain transport of drugs in man a reality. J. Pharm. Pharmacol. 56:3–17 (2004).
R. G. Thorne, G. J. Pronk, V. Padmanabhan, and W. H. Frey II. Delivery of insulin-like growth factor-1 top the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 127:481–496 (2004).
D. B. Judd, M. D. Dowle, D. Middlemiss, D. I. Scopes, B. C. Ross, T. I. Jack, M. Pass, E. Tranquillini, J. E. Hobson, T. A. Panchal, P. Stuart, J. M. S. Paton, T. Hubbard, A. Hilditch, G. M. Drew, M. J. Robertson, K. L. Clark, A. Travers, A. A. E. Hunt, J. Polley, P. J. Eddershaw, M. K. Bayliss, G. R. Manches, M. D. Donelly, D. G. Walker, and S. A. Richards. Bromobenzofuran-based non-peptide antagonists of angiotensin LL: GR138950, a potent antihypertensive agent with high oral bioavailability. J. M. Chem. 16:3108–3120 (1994).
S. Hirai, T. Yashiki, T. Matsuzawa, and H. Mima. Absorption of drugs from the nasal mucosa of rat. Int. J. Pharm. 7:317–325 (1981).
A. N. Fisher, K. Brown, S. S. Davis, G. D. Parr, and D. A. Smith. The nasal absorption of sodium cromoglycate in the albino-rat. J. Pharm. Pharmacol. 37:38–41 (1985).
S. T. Charlton, S. S. Davis, and L. Illum. Nasal administration of an angiotensin antagonist in the rat model: Effect of bioadhesive formulations on the distribution of drugs to the systemic and central nervous systems. Int. J. Pharm. 338:94–103 (2007).
M. P. Van der Berg, P. Merkus, S. G. Romeijn, J. Coos Verhoef, and F. W. H. M. Merkus. Hydroxycobalamin uptake into the cerebrospinal fluid after nasal and intravenous delivery in rats and humans. J. Drug Target. 11:325–331 (2003).
Q. Zhang, X. Jiang, W. Jiang, W. Lu, L. Su, and Z. Shi. Prepration of nimodine-loaded microsemulsion for intranasal delivery and evaluation of the targeting efficiency to brain. Int. J. Pharm. 275:85–96 (2004).
Laboratory Animal Science Association. Collection of Blood Samples. LASA, Tamworth, UK, 1998.
H. Davson, K. Welch, and M. B. Segal. Secretion of cerebrospinal fluid. In: The Physiology and Pathophysiology of the Cerebrospinal Fluid, Churchill Livingstone, London (1987).
T. Sakane, M. Akizuki, S. Yamashita, H. Sezaki, and T. Nagai. Direct drug transport from the rat nasal cavity to the cerebrospinal fluid: the relation to the dissociation of the drug. J. Pharm. Pharmacol. 46:378–379 (1994).
W. M. Boek, K. Graamans, and E. M. Huizing. Ciliary beat frequency of human SPHENOID SINUS MUCOSA AFTER CRYPRESERVATION. Eur. Arch. Otorhinolaryngol. 255:135–137 (1998).
R. U. Agu, M. Jorissen, T. Willems, R. Kinget, and N. Verbeke. Mechanistic appraisal of the effects of some protease inhibitors on ciliary beat frequency in a sequential cell culture system of human nasal epithelium. Eur. J. Pharm. Biopharm. 55:283–289 (2003).
N. H. Kleinsasser, J. Juchhoff, B. C. Wallner, A. Bergner, U. A. Harreus, F. Gamarra, M. Buhrlen, R. M. Huber, and A. W. Rettenmeiser. The use of mini-organ cultures of human upper aerodigestive tract epithelia in ecogenotoxicology. Mut. Res. 561:63–73 (2004).
W. M. Faber. The nasal mucosa and the subarachnoid space. Am.J.Anat. 62:121–148 (1937).
T. Sakane, M. Akizuki, Y. Taki, S. Yamashita, H. Sezaki, and T. Nadai. Direct drug transport from the rat nasal cavity to the cerebrospinal fluid: The relation to the molecular weight of drugs. J. Pharm. Pharmacol. 47:379–381 (1995).
M. Dahlin, U. Bergman, B. Jansson, E. Bjork, and E. Brittebo. Transfer of dopamine in the olfactory pathway following nasal administration in mice. Pharm.Res. 17:737–742 (2000).
C. Eriksson, U. Bergman, A. Franzen, M. Sjoblom, and E. B. Brittebo. Transfer of some carboxylic acids in the olfactory system following intranasal administration. J. Drug Target. 7:131–142 (1999).
Acknowledgements
Thanks are extended to GlaxoSmithKline for the provision of a Case award to support this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
To be submitted to: Pharmaceutical Research
Rights and permissions
About this article
Cite this article
Charlton, S.T., Whetstone, J., Fayinka, S.T. et al. Evaluation of Direct Transport Pathways of Glycine Receptor Antagonists and an Angiotensin Antagonist from the Nasal Cavity to the Central Nervous System in the Rat Model. Pharm Res 25, 1531–1543 (2008). https://doi.org/10.1007/s11095-008-9550-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9550-2