Purpose
Eritoran (E5564) is a glycophospholipid that acts as a toll-like receptor 4 (TLR4) antagonist that is being tested as a treatment for severe sepsis and septic shock. In the blood, eritoran binds to plasma lipoproteins altering its pharmacokinetic and pharmacodynamic (PD) effects in vivo. The purpose of this study was to determine the influence of changes in plasma cholesterol and triglyceride concentrations on the plasma pharmacokinetics and ex vivo activity of eritoran following single intravenous bolus dosing of eritoran to healthy female rabbits fed either a regular chow diet or a cholesterol-enriched diet. This was done with eritoran administered as stable micelle formulations of mean hydrodynamic diameters of 8 or 27 nm).
Methods
Female New Zealand White rabbits were fed a standard diet for 7 days and then randomly assigned either a regular chow diet [regular-diet (n = 9)] or a cholesterol-enriched diet [cholesterol-diet (n = 12)] for an additional 7 days. Following feeding of these diets a single intravenous bolus dose of eritoran (0.5 mg/kg) formulated into either “small micelles” (8 nm in diameter) or “large micelles” (27 nm in diameter) was administered to regular-fed and cholesterol-fed rabbits. Serial blood samples were obtained prior to eritoran administration and at the following times post injection: 0.083 (5 min), 1, 2, 4, 8, 10, 24, 48 and 72 h. Plasma was analyzed for eritoran concentrations using LC/MS/MS. Total plasma cholesterol (TC) and triglyceride (TG) levels were quantified using enzymatic kits. Plasma eritoran pharmacokinetic (PK) parameters were estimated by non-compartmental analysis using the WinNonlin nonlinear estimation program. To analyze PD activity, whole blood obtained at 0.083 (5 min), 2, 24, 48 and 72 h following eritoran administration was assessed for ex vivo activity by measuring the ability of 1 and 10 ng/ml LPS to elicit TNF-α release.
Results
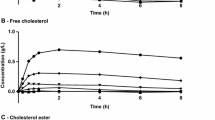
Total plasma cholesterol and triglyceride levels were significantly higher in cholesterol-fed rabbits compared to the rabbits fed a regular chow diet. Diet had no effect on the estimated plasma PK parameters. However, PD activity of both small and large micelle eritoran as measured by an ex vivo challenge dose of 1 ng/ml LPS was reduced in blood of cholesterol-fed rabbits compared to normal-fed rabbits. Comparison of PK parameters for small and large micelles indicated that small micelles had increased AUC0–72 h, decreased plasma clearance and increased initial concentration (measured at 5 min post administration) compared to the large micelle formulation. Consistent with this observation, eritoran formulated into small micelles had significantly greater ex vivo activity than large micelles and was independent of TC and TG concentrations.
Conclusions
These findings suggest that plasma pharmacokinetics and activity of eritoran maybe influenced by eritoran micelle size and plasma TC and TG concentrations.




Similar content being viewed by others
References
X. Du, A. Poltorak, M. Silva, and B. Beutler. Analysis of Tlr4-mediated LPS signal transduction in macrophages by mutational modification of the receptor. Blood Cells Mol. Dis. 25:328–338 (1999).
S. K. Huang, K. D. Lee, K. Hong, D. S. Friend, and D. Papahadjopoulos Microscopic localization of sterically stabilized liposomes in colon-carcinoma-bearing mice. Cancer Res. 52:5135–5143 (1992).
K. Kaneko, R. Ueda, K. Kikuchi, Y. Sano, and T. Yoshimura. Quantitative determination of a potent lipopolysaccharide antagonist, E5564, in rat and dog plasma by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B Biomed. Sci. Appl. 736:67–75 (1999).
M. Lynn, D. P. Rossignol, J. L. Wheeler, R. J. Kao, C. A. Perdomo, R. Noveck, R. Vargas, T. D’Angelo, S. Gotzkowsky, and F. G. McMahon. Blocking of responses to endotoxin by E5564 in healthy volunteers with experimental endotoxemia. J. Infect. Dis. 187:631–639 (2003).
M. Mullarkey, J. R. Rose, J. Bristol, T. Kawata, A. Kimura, S. Kobayashi, M. Przetak, J. Chow, F. Gusovsky, W. J. Christ, and D. P. Rossignol. Inhibition of endotoxin response by E5564, a novel toll-like receptor 4-directed endotoxin antagonist. J. Pharmacol. Exp. Ther. 304:1093–1102 (2003).
F. Norido, A. Zatta, C. Fiorito, M. Prosdocimi, and G. Weber. Hematologic and biochemical analysis profiles of selectively bred WHHL rabbits. Lab. Anim. Sci. 43:319–323 (1993).
N. M. O’Meara, R. A. Devery, D. Owens, P. B. Collins, A. H. Johnson, and G. H. Tomkin. Serum lipoproteins and cholesterol metabolism in two hypercholesterolemic rabbit models. Diabetologia. 34:139–143 (1991).
S. M. Opal, P. J. Scannon, J. L. Vincent, et al. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J. Infect. Dis. 180:1584–1589 (1999).
A. Poltorak, X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 282:2085–2088 (1998).
M. Ramaswamy, T. L. Wallace, P. A. Cossum, and K. M. Wasan. Species differences in the proportion of plasma lipoprotein lipid carried by high-density lipoproteins influence the distribution of free and liposomal nystatin in human, dog, and rat plasma. Antimicrob. Agents Chemother. 43:1424–1428 (1999).
J. R. Rose, W. J. Christ, J. R. Bristol, T. Kawata, and D. P. Rossignol. Agonistic and antagonistic activities of bacterially derived Rhodobacter sphaeroides lipid A: comparison with activities of synthetic material of the proposed structure and analogs. Infect. Immun. 63:833–839 (1995).
J. R. Rose, M. A. Mullarkey, W. J. Christ, L. D. Hawkins, M. Lynn, Y. Kishi, K. M. Wasan, K. Peteherych, and D. P. Rossignol. Consequences of interaction of a lipophilic endotoxin antagonist with plasma lipoproteins. Antimicrob. Agents Chemother. 44:504–510 (2000).
D. P. Rossignol, W. J. Christ, L. D. Hawkins, S. Kobayashi, T. Kawata, M. Lynn, I. Yamatsu, and Y. Kishi. Synthetic endotoxin antagonists. In H. Brade, S. M. Opal, S. N. Vogel, and D. C. Morrison (eds.), Endotoxin in Health and Disease. Marcel Dekker, New York, 1999, pp. 699–717.
D. P. Rossignol, and M. Lynn. Antagonism of in vivo and ex vivo response to endotoxin by E5564, a novel synthetic lipid A antagonist. J. Endotoxin Res. 8:483–488 (2003).
D. P. Rossignol, K. M. Wasan, E. Choo, E. Yau, N. Wong, J. Rose, J. Moran, and M. Lynn. Safety, pharmacokinetics, pharmacodynamics, and plasma lipoprotein distribution of eritoran (E5564) during continuous intravenous infusion into healthy volunteers. Antimicrob. Agents Chemother. 48:3233–3240 (2004).
A. Suganuma, D. P. Rossignol, K. Kaneko, A. W. LaRochelle, and W. D. Kerns. Clinical pharmacology of E5564, a lipid A antagonist, in a canine lipopolysaccharide challenge model. J. Endotoxin Res. 6:115 (2000).
T. J. Walsh, J. Bacher, and P. A. Pizzo. Chronic silastic central venous catherization for reduction, maintenance and support of persistent granulocytopenia in rabbits. Lab. Anim. Sci. 38:467–471 (1988).
K. M. Wasan, and S. M. Cassidy. Role of plasma lipoproteins in modifying the biological activity of hydrophobic drugs. J. Pharm. Sci. 87:411–424 (1998).
K. M. Wasan, S. M. Cassidy, M. Ramaswamy, A. Kennedy, F. W. Strobel, S. P. Ng, and T. Y. Lee. A comparison of step-gradient and sequential density ultracentrifugation and the use of lipoprotein deficient plasma controls in determining the plasma lipoprotein distribution of lipid-associated nystatin and cyclosporine. Pharm. Res. 16:165–169 (1999a).
K. M. Wasan, P. H. Pritchard, M. Ramaswamy, W. Wong, E. M. Donnachie, and L. J. Brunner. Differences in lipoprotein lipid concentration and composition modify the plasma distribution of cyclosporine. Pharm. Res. 14:1613–1620 (1997).
K. M. Wasan, O. Sivak, R. Cote, A. M. MacInnes, K. D. Boulanger, M. Lynn, W. J. Christ, L. D. Hawkins, and D. P. Rossignol. Association of E5564, an endotoxin antagonist, with high-density lipoproteins in vitro: dependence on low-density and triglyceride-rich lipoprotein concentration. Antimicrob. Agents Chemother. 47:2796–2803 (2003).
K. M. Wasan, F. W. Strobel, S. C. Parrott, M. Lynn, W. J. Christ, L. D. Hawkins, and D. P. Rossignol. Lipoprotein distribution of a novel endotoxin antagonist, E5531, in plasma from human subjects with various lipid levels. Antimicrob. Agents Chemother. 43:2562–2564 (1999b).
Y. N. Wong, D. Rossignol, J. R. Rose, R. Kao, A. Carter, and M. Lynn. Safety, pharmacokinetics and pharmacodynamics of E5564, a lipid A antagonist, during an ascending single-dose clinical study. J. Clin. Pharmacol. 43:735–742 (2003).
J. H. Zar. Multiple regression and correlation in biostatistical analysis. Prentice-Halls, Englewood, NJ, 1984.
Acknowledgements
This study was supported by funding from Eisai Medical Research to KMW. The authors would like to thank Miguel Pacheco and Julian Kay of the Acute Care Animal Unit at the University of British Columbia for their surgical assistance and Deanna Knighton from ZeptoMetrix Corporation for completing the TNF-α assay.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wasan, K.M., Risovic, V., Sivak, O. et al. Influence of Plasma Cholesterol and Triglyceride Concentrations and Eritoran (E5564) Micelle Size on its Plasma Pharmacokinetics and Ex Vivo Activity Following Single Intravenous Bolus Dose Into Healthy Female Rabbits. Pharm Res 25, 176–182 (2008). https://doi.org/10.1007/s11095-007-9428-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9428-8