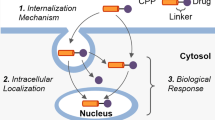
Abstract
Cell penetrating peptides, generally categorized as amphipathic or cationic depending on their sequence, are increasingly drawing attention as a non-invasive delivery technology for macromolecules. Delivery of a diverse set of cargo in terms of size and nature ranging from small molecules to particulate cargo has been attempted using different types of cell penetrating peptides (CPPs) in vitro and in vivo. However, the internalization mechanism of CPPs is an unresolved issue to date, with dramatic changes in view regarding the involvement of endocytosis as a pathway of internalization. A key reason for the lack of consensus on the mechanism can be attributed to the methodology in deciphering the internalization mechanism. In this review, we highlight some of the methodology concerns, focus more on the internalization pathway and also provide a novel perspective about the intracellular processing of CPPs, which is a crucial aspect to consider when selecting a cell penetrating peptide as a drug delivery system. In addition, recent applications of cell penetrating peptides for the delivery of small molecules, peptides, proteins, oligonucleotides, nanoparticles and liposomes have been reviewed.


Similar content being viewed by others
Abbreviations
- CHO cells:
-
Chinese hamster ovary cells
- CLIO:
-
cross-linked iron oxide nanoparticles
- CPPs:
-
cell penetrating peptides
- EGFP:
-
enhanced green fluorescent protein
- GAGs:
-
glycosaminoglycans
- GFP:
-
green fluorescent protein
- HIV-1:
-
human immunodeficiency virus–1
- MAP:
-
model amphipathic peptides
- MTPs:
-
membrane transduction peptides
- MTS:
-
mitochondrial targeting signal
- NPC:
-
nuclear pore complex
- PCI:
-
photochemical internalization
- PNA:
-
peptide nucleic acid
- PTDs:
-
protein transduction domains
- USPIO:
-
ultra small paramagnetic iron oxide nanoparticles.
References
S. Futaki. Arginine-rich peptides: potential for intracellular delivery of macromolecules and the mystery of the translocation mechanisms. Int. J. Pharm. 245:1–7 (2002).
J. Fernandez-Carneado, M. J. Kogan, S. Pujals, and E. Giralt. Amphipathic peptides and drug delivery. Biopolymers 76:196–203 (2004).
R. Trehin and H. P. Merkle. Chances and pitfalls of cell penetrating peptides for cellular drug delivery. Eur. J. Pharm. Biopharm. 58:209–223 (2004).
J. L. Zaro and W. C. Shen. Quantitative comparison of membrane transduction and endocytosis of oligopeptides. Biochem. Biophys. Res. Commun. 307:241–247 (2003).
P. A. Wender, J. B. Rothbard, T. C. Jessop, E. L. Kreider, and B. L. Wylie. Oligocarbamate molecular transporters: design, synthesis, and biological evaluation of a new class of transporters for drug delivery. J. Am. Chem. Soc. 124:13382–13383 (2002).
S. Futaki, I. Nakase, T. Suzuki, Z. Youjun, and Y. Sugiura. Translocation of branched-chain arginine peptides through cell membranes: flexibility in the spatial disposition of positive charges in membrane-permeable peptides. Biochemistry 41:7925–7930 (2002).
S. Futaki, T. Suzuki, W. Ohashi, T. Yagami, S. Tanaka, K. Ueda, and Y. Sugiura. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J. Biol. Chem. 276:5836–5840 (2001).
P. A. Wender, D. J. Mitchell, K. Pattabiraman, E. T. Pelkey, L. Steinman, and J. B. Rothbard. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc. Natl. Acad. Sci. U. S. A. 97:13003–13008 (2000).
D. J. Mitchell, D. T. Kim, L. Steinman, C. G. Fathman, and J. B. Rothbard. Polyarginine enters cells more efficiently than other polycationic homopolymers. J. Pept. Res. 56:318–325 (2000).
B. J. Calnan, B. Tidor, S. Biancalana, D. Hudson, and A. D. Frankel. Arginine-mediated RNA recognition: the arginine fork. Science 252:1167–1171 (1991).
J. Oehlke, A. Scheller, B. Wiesner, E. Krause, M. Beyermann, E. Klauschenz, M. Melzig, and M. Bienert. Cellular uptake of an alpha-helical amphipathic model peptide with the potential to deliver polar compounds into the cell interior non-endocytically. Biochim. Biophys. Acta. 1414:127–139 (1998).
A. Scheller, J. Oehlke, B. Wiesner, M. Dathe, E. Krause, M. Beyermann, M. Melzig, and M. Bienert. Structural requirements for cellular uptake of alpha-helical amphipathic peptides. J. Pept. Sci. 5:185–194 (1999).
T. Suzuki, S. Futaki, M. Niwa, S. Tanaka, K. Ueda, and Y. Sugiura. Possible existence of common internalization mechanisms among arginine-rich peptides. J. Biol. Chem. 277:2437–2443 (2002).
E. Vives, P. Brodin, and B. Lebleu. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J. Biol. Chem. 272:16010–16017 (1997).
D. Derossi, S. Calvet, A. Trembleau, A. Brunissen, G. Chassaing, and A. Prochiantz. Cell internalization of the third helix of the Antennapedia homeodomain is receptor-independent. J. Biol. Chem. 271:18188–18193 (1996).
J. P. Richard, K. Melikov, E. Vives, C. Ramos, B. Verbeure, M. J. Gait, L. V. Chernomordik, and B. Lebleu. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J. Biol. Chem. 278:585–590 (2003).
J. P. Richard, K. Melikov, H. Brooks, P. Prevot, B. Lebleu, and L. V. Chernomordik. Cellular uptake of unconjugated TAT peptide involves clathrin-dependent endocytosis and heparan sulfate receptors. J. Biol. Chem. 280:15300–15306 (2005).
I. M. Kaplan, J. S. Wadia, and S. F. Dowdy. Cationic TAT peptide transduction domain enters cells by macropinocytosis. J. Control. Release 102:247–253 (2005).
I. Nakase, M. Niwa, T. Takeuchi, K. Sonomura, N. Kawabata, Y. Koike, M. Takehashi, S. Tanaka, K. Ueda, J. C. Simpson, A. T. Jones, Y. Sugiura, and S. Futaki. Cellular uptake of arginine-rich peptides: roles for macropinocytosis and actin rearrangement. Mol. Ther. 10:1011–1022 (2004).
R. Fischer, K. Kohler, M. Fotin-Mleczek, and R. Brock. A stepwise dissection of the intracellular fate of cationic cell-penetrating peptides. J. Biol. Chem. 279:12625–12635 (2004).
T. B. Potocky, A. K. Menon, and S. H. Gellman. Cytoplasmic and nuclear delivery of a TAT-derived peptide and a beta-peptide after endocytic uptake into HeLa cells. J. Biol. Chem. 278:50188–50194 (2003).
J. S. Wadia, R. V. Stan, and S. F. Dowdy. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat. Med. 10:310–315 (2004).
A. Fittipaldi, A. Ferrari, M. Zoppe, C. Arcangeli, V. Pellegrini, F. Beltram, and M. Giacca. Cell membrane lipid rafts mediate caveolar endocytosis of HIV-1 Tat fusion proteins. J. Biol. Chem. 278:34141–34149 (2003).
S. Console, C. Marty, C. Garcia-Echeverria, R. Schwendener, and K. Ballmer-Hofer. Antennapedia and HIV transactivator of transcription (TAT) “protein transduction domains” promote endocytosis of high molecular weight cargo upon binding to cell surface glycosaminoglycans. J. Biol. Chem. 278:35109–35114 (2003).
J. C. Mai, H. Shen, S. C. Watkins, T. Cheng, and P. D. Robbins. Efficiency of protein transduction is cell type-dependent and is enhanced by dextran sulfate. J. Biol. Chem. 277:30208–30218 (2002).
C. H. Tung and R. Weissleder. Arginine containing peptides as delivery vectors. Adv. Drug. Deliv. Rev. 55:281–294 (2003).
J. L. Zaro and W. C. Shen. Evidence that membrane transduction of oligoarginine does not require vesicle formation. Exp. Cell. Res. 307:164–173 (2005).
J. A. Leifert, S. Harkins, and J. L. Whitton. Full-length proteins attached to the HIV tat protein transduction domain are neither transduced between cells, nor exhibit enhanced immunogenicity. Gene Ther. 9:1422–1428 (2002).
A. Astriab-Fisher, D. Sergueev, M. Fisher, B. R. Shaw, and R. L. Juliano. Conjugates of antisense oligonucleotides with the Tat and antennapedia cell-penetrating peptides: effects on cellular uptake, binding to target sequences, and biologic actions. Pharm. Res. 19:744–754 (2002).
P. O. Falnes, J. Wesche, and S. Olsnes. Ability of the Tat basic domain and VP22 to mediate cell binding, but not membrane translocation of the diphtheria toxin A-fragment. Biochemistry 40:4349–4358 (2001).
A. Eguchi, T. Akuta, H. Okuyama, T. Senda, H. Yokoi, H. Inokuchi, S. Fujita, T. Hayakawa, K. Takeda, M. Hasegawa, and M. Nakanishi. Protein transduction domain of HIV-1 Tat protein promotes efficient delivery of DNA into mammalian cells. J. Biol. Chem. 276:26204–26210 (2001).
G. Drin, S. Cottin, E. Blanc, A. R. Rees, and J. Temsamani. Studies on the internalization mechanism of cationic cell-penetrating peptides. J. Biol. Chem. 278:31192–31201 (2003).
M. Zhao and R. Weissleder. Intracellular cargo delivery using tat peptide and derivatives. Med. Res. Rev. 24:1–12 (2004).
H. J. Lee and W. M. Pardridge. Pharmacokinetics and delivery of tat and tat-protein conjugates to tissues in vivo. Bioconjug. Chem. 12:995–999 (2001).
A. Ho, S. R. Schwarze, S. J. Mermelstein, G. Waksman, and S. F. Dowdy. Synthetic protein transduction domains: enhanced transduction potential in vitro and in vivo. Cancer Res. 61:474–477 (2001).
S. R. Schwarze, K. A. Hruska, and S. F. Dowdy. Protein transduction: unrestricted delivery into all cells? Trends Cell. Biol. 10:290–295 (2000).
J. B. Rothbard, S. Garlington, Q. Lin, T. Kirschberg, E. Kreider, P. L. McGrane, P. A. Wender, and P. A. Khavari. Conjugation of arginine oligomers to cyclosporin A facilitates topical delivery and inhibition of inflammation. Nat. Med. 6:1253–1257 (2000).
M. Lewin, N. Carlesso, C. H. Tung, X. W. Tang, D. Cory, D. T. Scadden, and R. Weissleder. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat. Biotechnol. 18:410–414 (2000).
S. R. Schwarze, A. Ho, A. Vocero-Akbani, and S. F. Dowdy. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science 285:1569–1572 (1999).
V. Polyakov, V. Sharma, J. L. Dahlheimer, C. M. Pica, G. D. Luker, and D. Piwnica-Worms. Novel Tat-peptide chelates for direct transduction of technetium-99 m and rhenium into human cells for imaging and radiotherapy. Bioconjug. Chem. 11:762–771 (2000).
B. X. Chen and B. F. Erlanger. Intracellular delivery of monoclonal antibodies. Immunol. Lett. 84:63–67 (2002).
J. L. Zaro and W. C. Shen. Cytosolic delivery of a p16-peptide oligoarginine conjugate for inhibiting proliferation of MCF7 cells. J. Control. Release 108:409–417 (2005).
M. Silhol, M. Tyagi, M. Giacca, B. Lebleu, and E. Vives. Different mechanisms for cellular internalization of the HIV-1 Tat-derived cell penetrating peptide and recombinant proteins fused to Tat. Eur. J. Biochem. 269:494–501 (2002).
P. E. Thoren, D. Persson, P. Isakson, M. Goksor, A. Onfelt, and B. Norden. Uptake of analogs of penetratin, Tat(48–60) and oligoarginine in live cells. Biochem. Biophys. Res. Commun. 307:100–107 (2003).
A. Elmquist and U. Langel. In vitro uptake and stability study of pVEC and its all-D analog. Biol. Chem. 384:387–393 (2003).
D. Terrone, S. L. Sang, L. Roudaia, and J. R. Silvius. Penetratin and related cell-penetrating cationic peptides can translocate across lipid bilayers in the presence of a transbilayer potential. Biochemistry 42:13787–13799 (2003).
H. Binder and G. Lindblom. Charge-dependent translocation of the Trojan peptide penetratin across lipid membranes. Biophys. J. 85:982–995 (2003).
H. Nagahara, A. M. Vocero-Akbani, E. L. Snyder, A. Ho, D. G. Latham, N. A. Lissy, M. Becker-Hapak, S. A. Ezhevsky, and S. F. Dowdy. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat. Med. 4:1449–1452 (1998).
A. Prochiantz. Messenger proteins: homeoproteins, TAT and others. Curr. Opin. Cell. Biol. 12:400–406 (2000).
A. Prochiantz. Homeodomain-derived peptides. In and out of the cells. Ann. N. Y. Acad. Sci. 886:172–179 (1999).
A. Ziegler, X. L. Blatter, A. Seelig, and J. Seelig. Protein transduction domains of HIV-1 and SIV TAT interact with charged lipid vesicles. Binding mechanism and thermodynamic analysis. Biochemistry 42:9185–9194 (2003).
Z. Salamon, G. Lindblom, and G. Tollin. Plasmon-waveguide resonance and impedance spectroscopy studies of the interaction between penetratin and supported lipid bilayer membranes. Biophys. J. 84:1796–1807 (2003).
T. Takeuchi, M. Kosuge, A. Tadokoro, Y. Sugiura, M. Nishi, M. Kawata, N. Sakai, S. Matile, and S. Futaki. Direct and rapid cytosolic delivery using cell-penetrating peptides mediated by pyrenebutyrate. ACS Chem. Biol. 1:299–303 (2006).
N. Sakai, T. Takeuchi, S. Futaki, and S. Matile. Direct observation of anion-mediated translocation of fluorescent oligoarginine carriers into and across bulk liquid and anionic bilayer membranes. Chembiochem. 6:114–122 (2005).
N. Sakai and S. Matile. Anion-mediated transfer of polyarginine across liquid and bilayer membranes. J. Am. Chem. Soc. 125:14348–14356 (2003).
A. Ferrari, V. Pellegrini, C. Arcangeli, A. Fittipaldi, M. Giacca, and F. Beltram. Caveolae-mediated internalization of extracellular HIV-1 tat fusion proteins visualized in real time. Mol. Ther. 8:284–294 (2003).
S. D. Conner and S. L. Schmid. Regulated portals of entry into the cell. Nature 422:37–44 (2003).
I. A. Khalil, K. Kogure, S. Futaki, and H. Harashima. High density of octaarginine stimulates macropinocytosis leading to efficient intracellular trafficking for gene expression. J. Biol. Chem. 281:3544–3551 (2006).
M. Fretz, J. Jin, R. Conibere, N. A. Penning, S. Al-Taei, G. Storm, S. Futaki, T. Takeuchi, I. Nakase, and A. T. Jones. Effects of Na(+)/H(+) exchanger inhibitors on subcellular localisation of endocytic organelles and intracellular dynamics of protein transduction domains HIV-TAT peptide and octaarginine. J. Control. Release 116:247–254 (2006).
V. P. Torchilin, R. Rammohan, V. Weissig, and T. S. Levchenko. TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proc. Natl. Acad. Sci. U. S. A. 98:8786–8791 (2001).
J. L. Zaro, T. E. Rajapaksa, C. T. Okamoto, and W. C. Shen. Membrane transduction of oligoarginine in HeLa cells is not mediated by macropinocytosis. Mol. Pharm. 3:181–186 (2006).
P. Saalik, A. Elmquist, M. Hansen, K. Padari, K. Saar, K. Viht, U. Langel, and M. Pooga. Protein cargo delivery properties of cell-penetrating peptides. A comparative study. Bioconjug. Chem. 15:1246–1253 (2004).
M. Tyagi, M. Rusnati, M. Presta, and M. Giacca. Internalization of HIV-1 tat requires cell surface heparan sulfate proteoglycans. J. Biol. Chem. 276:3254–3261 (2001).
M. Rusnati, G. Tulipano, D. Spillmann, E. Tanghetti, P. Oreste, G. Zoppetti, M. Giacca, and M. Presta. Multiple interactions of HIV-I Tat protein with size-defined heparin oligosaccharides. J. Biol. Chem. 274:28198–28205 (1999).
M. Rusnati, G. Tulipano, C. Urbinati, E. Tanghetti, R. Giuliani, M. Giacca, M. Ciomei, A. Corallini, and M. Presta. The basic domain in HIV-1 Tat protein as a target for polysulfonated heparin-mimicking extracellular Tat antagonists. J. Biol. Chem. 273:16027–16037 (1998).
M. Rusnati, D. Coltrini, P. Oreste, G. Zoppetti, A. Albini, D. Noonan, F. d’Adda di Fagagna, M. Giacca, and M. Presta. Interaction of HIV-1 Tat protein with heparin. Role of the backbone structure, sulfation, and size. J. Biol. Chem. 272:11313–11320 (1997).
S. M. Fuchsand R. T. Raines. Pathway for polyarginine entry into mammalian cells. Biochemistry 43:2438–2444 (2004).
S. Sandgren, F. Cheng, and M. Belting. Nuclear targeting of macromolecular polyanions by an HIV-Tat derived peptide. Role for cell-surface proteoglycans. J. Biol. Chem. 277:38877–38883 (2002).
E. Goncalves, E. Kitas, and J. Seelig. Binding of oligoarginine to membrane lipids and heparan sulfate: structural and thermodynamic characterization of a cell-penetrating peptide. Biochemistry 44:2692–2702 (2005).
A. Ziegler and J. Seelig. Interaction of the protein transduction domain of HIV-1 TAT with heparan sulfate: binding mechanism and thermodynamic parameters. Biophys. J. 86:254–263 (2004).
S. Hakansson and M. Caffrey. Structural and dynamic properties of the HIV-1 tat transduction domain in the free and heparin-bound states. Biochemistry 42:8999–9006 (2003).
M. Green and P. M. Loewenstein. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell 55:1179–1188 (1988).
A. D. Frankel and C. O. Pabo. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 55:1189–1193 (1988).
W. C. Shen and H. J. Ryser. Conjugation of poly-L-lysine to albumin and horseradish peroxidase: a novel method of enhancing the cellular uptake of proteins. Proc. Natl. Acad. Sci. U. S. A. 75:1872–1876 (1978).
H. J. Ryser and W. C. Shen. Conjugation of methotrexate to poly(L-lysine) increases drug transport and overcomes drug resistance in cultured cells. Proc. Natl. Acad. Sci. U. S. A. 75:3867–3870 (1978).
M. Yanagishita and V. C. Hascall. Cell surface heparan sulfate proteoglycans. J. Biol. Chem. 267:9451–9454 (1992).
J. A. Swanson and C. Watts. Macropinocytosis. Trends Cell. Biol. 5:424–428 (1995).
H. Michiue, K. Tomizawa, F. Y. Wei, M. Matsushita, Y. F. Lu, T. Ichikawa, T. Tamiya, I. Date, and H. Matsui. The NH2 terminus of influenza virus hemagglutinin-2 subunit peptides enhances the antitumor potency of polyarginine-mediated p53 protein transduction. J. Biol. Chem. 280:8285–8289 (2005).
J. R. Maiolo, 3rd, E. A. Ottinger, and M. Ferrer. Specific redistribution of cell-penetrating peptides from endosomes to the cytoplasm and nucleus upon laser illumination. J. Am. Chem. Soc. 126:15376–15377 (2004).
T. Shiraishi and P. E. Nielsen. Photochemically enhanced cellular delivery of cell penetrating peptide-PNA conjugates. FEBS Lett. 580:1451–1456 (2006).
D. Lechardeur and G. L. Lukacs. Nucleocytoplasmic transport of plasmid DNA: a perilous journey from the cytoplasm to the nucleus. Hum. Gene. Ther. 17:882–889 (2006).
A. Ziegler, P. Nervi, M. Durrenberger, and J. Seelig. The cationic cell-penetrating peptide CPP(TAT) derived from the HIV-1 protein TAT is rapidly transported into living fibroblasts: optical, biophysical, and metabolic evidence. Biochemistry 44:138–148 (2005).
M. Zorko and U. Langel. Cell-penetrating peptides: mechanism and kinetics of cargo delivery. Adv. Drug. Deliv. Rev. 57:529–545 (2005).
R. Truant and B. R. Cullen. The arginine-rich domains present in human immunodeficiency virus type 1 Tat and Rev function as direct importin beta-dependent nuclear localization signals. Mol. Cell Biol. 19:1210–1217 (1999).
W. J. Gehring, Y. Q. Qian, M. Billeter, K. Furukubo-Tokunaga, A. F. Schier, D. Resendez-Perez, M. Affolter, G. Otting, and K. Wuthrich. Homeodomain-DNA recognition. Cell 78:211–223 (1994).
J. Adams. The proteasome: structure, function, and role in the cell. Cancer Treat. Rev. 29 (Suppl 1):3–9 (2003).
M. H. Glickman and A. Ciechanover. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol. Rev. 82:373–428 (2002).
E. Geier, G. Pfeifer, M. Wilm, M. Lucchiari-Hartz, W. Baumeister, K. Eichmann, and G. Niedermann. A giant protease with potential to substitute for some functions of the proteasome. Science 283:978–981 (1999).
R. Trehin, H. M. Nielsen, H. G. Jahnke, U. Krauss, A. G. Beck-Sickinger, and H. P. Merkle. Metabolic cleavage of cell-penetrating peptides in contact with epithelial models: human calcitonin (hCT)-derived peptides, Tat(47–57) and penetratin(43–58). Biochem. J. 382:945–956 (2004).
D. L. Kolson, R. Collman, R. Hrin, J. W. Balliet, M. Laughlin, K. A. McGann, C. Debouck, and F. Gonzalez-Scarano. Human immunodeficiency virus type 1 Tat activity in human neuronal cells: uptake and trans-activation. J. Gen. Virol. 75 (Pt 8):1927–1934 (1994).
M. E. Lindgren, M. M. Hallbrink, A. M. Elmquist, and U. Langel. Passage of cell-penetrating peptides across a human epithelial cell layer in vitro. Biochem. J. 377:69–76 (2004).
S. Violini, V. Sharma, J. L. Prior, M. Dyszlewski, and D. Piwnica-Worms. Evidence for a plasma membrane-mediated permeability barrier to Tat basic domain in well-differentiated epithelial cells: lack of correlation with heparan sulfate. Biochemistry 41:12652–12661 (2002).
R. Trehin, U. Krauss, A. G. Beck-Sickinger, H. P. Merkle, and H. M. Nielsen. Cellular uptake but low permeation of human calcitonin-derived cell penetrating peptides and Tat(47–57) through well-differentiated epithelial models. Pharm. Res. 21:1248–1256 (2004).
J. F. Liang and V. C. Yang. Insulin-cell penetrating peptide hybrids with improved intestinal absorption efficiency. Biochem. Biophys. Res. Commun. 335:734–738 (2005).
A. M. Koch, F. Reynolds, H. P. Merkle, R. Weissleder, and L. Josephson. Transport of surface-modified nanoparticles through cell monolayers. Chembiochem 6:337–345 (2005).
C. Rousselle, P. Clair, J. M. Lefauconnier, M. Kaczorek, J. M. Scherrmann, and J. Temsamani. New advances in the transport of doxorubicin through the blood-brain barrier by a peptide vector-mediated strategy. Mol. Pharmacol. 57:679–86 (2000).
G. P. Dietz, P. C. Valbuena, B. Dietz, K. Meuer, P. Mueller, J. H. Weishaupt, and M. Bahr. Application of a blood-brain-barrier-penetrating form of GDNF in a mouse model for Parkinson’s disease. Brain Res. 1082:61–66 (2006).
S. Santra, H. Yang, J. T. Stanley, P. H. Holloway, B. M. Moudgil, G. Walter, and R. A. Mericle. Rapid and effective labeling of brain tissue using TAT-conjugated CdS:Mn/ZnS quantum dots. Chem. Commun. (Camb) 3144–3146 (2005).
E. L. Snyder and S. F. Dowdy. Recent advances in the use of protein transduction domains for the delivery of peptides, proteins and nucleic acids in vivo. Expert Opin. Drug Deliv. 2:43–51 (2005).
G. P. Dietz and M. Bahr. Delivery of bioactive molecules into the cell: the Trojan horse approach. Mol. Cell. Neurosci. 27:85–131 (2004).
M. Lindgren, K. Rosenthal-Aizman, K. Saar, E. Eiriksdottir, Y. Jiang, M. Sassian, P. Ostlund, M. Hallbrink, and U. Langel. Overcoming methotrexate resistance in breast cancer tumour cells by the use of a new cell-penetrating peptide. Biochem. Pharmacol. 71:416–425 (2006).
L. Chaloin, P. Bigey, C. Loup, M. Marin, N. Galeotti, M. Piechaczyk, F. Heitz, and B. Meunier. Improvement of porphyrin cellular delivery and activity by conjugation to a carrier peptide. Bioconjug. Chem. 12:691–700 (2001).
J. Brunner and J. K. Barton. Targeting DNA mismatches with rhodium intercalators functionalized with a cell-penetrating peptide. Biochemistry 45:12295–12302 (2006).
C. J. Sherr. Principles of tumor suppression. Cell 116:235–246 (2004).
R. Fahraeus, S. Lain, K. L. Ball, and D. P. Lane. Characterization of the cyclin-dependent kinase inhibitory domain of the INK4 family as a model for a synthetic tumour suppressor molecule. Oncogene 16:587–596 (1998).
R. Hosotani, Y. Miyamoto, K. Fujimoto, R. Doi, A. Otaka, N. Fujii, and M. Imamura. Trojan p16 peptide suppresses pancreatic cancer growth and prolongs survival in mice. Clin. Cancer Res. 8:1271–1276 (2002).
C. Craig, M. Kim, E. Ohri, R. Wersto, D. Katayose, Z. Li, Y. H. Choi, B. Mudahar, S. Srivastava, P. Seth, and K. Cowan. Effects of adenovirus-mediated p16INK4A expression on cell cycle arrest are determined by endogenous p16 and Rb status in human cancer cells. Oncogene 16:265–272 (1998).
H. Y. Wu, K. Tomizawa, M. Matsushita, Y. F. Lu, S. T. Li, and H. Matsui. Poly-arginine-fused calpastatin peptide, a living cell membrane-permeable and specific inhibitor for calpain. Neurosci. Res. 47:131–135 (2003).
B. Law, L. Quinti, Y. Choi, R. Weissleder, and C. H. Tung. A mitochondrial targeted fusion peptide exhibits remarkable cytotoxicity. Mol. Cancer Ther. 5:1944–1949 (2006).
H. Yan, J. Thomas, T. Liu, D. Raj, N. London, T. Tandeski, S. A. Leachman, R. M. Lee, and D. Grossman. Induction of melanoma cell apoptosis and inhibition of tumor growth using a cell-permeable Survivin antagonist. Oncogene 25:6968–6974 (2006).
R. Diem, N. Taheri, G. P. Dietz, A. Kuhnert, K. Maier, M. B. Sattler, I. Gadjanski, D. Merkler, and M. Bahr. HIV-Tat-mediated Bcl-XL delivery protects retinal ganglion cells during experimental autoimmune optic neuritis. Neurobiol. Dis. 20:218–226 (2005).
M. F. Ross and M. P. Murphy. Cell-penetrating peptides are excluded from the mitochondrial matrix. Biochem. Soc. Trans. 32:1072–1074 (2004).
M. F. Ross, A. Filipovska, R. A. Smith, M. J. Gait, and M. P. Murphy. Cell-penetrating peptides do not cross mitochondrial membranes even when conjugated to a lipophilic cation: evidence against direct passage through phospholipid bilayers. Biochem. J. 383:457–468 (2004).
I. N. Shokolenko, M. F. Alexeyev, S. P. LeDoux, and G. L. Wilson. TAT-mediated protein transduction and targeted delivery of fusion proteins into mitochondria of breast cancer cells. DNA Repair (Amst) 4:511–518 (2005).
L. Cao, J. Si, W. Wang, X. Zhao, X. Yuan, H. Zhu, X. Wu, J. Zhu, and G. Shen. Intracellular localization and sustained prodrug cell killing activity of TAT-HSVTK fusion protein in hepatocelullar carcinoma cells. Mol. Cells. 21:104–111 (2006).
I. A. Ignatovich, E. B. Dizhe, A. V. Pavlotskaya, B. N. Akifiev, S. V. Burov, S. V. Orlov, and A. P. Perevozchikov. Complexes of plasmid DNA with basic domain 47–57 of the HIV-1 Tat protein are transferred to mammalian cells by endocytosis-mediated pathways. J. Biol. Chem. 278:42625–42636 (2003).
I. A. Khalil, S. Futaki, M. Niwa, Y. Baba, N. Kaji, H. Kamiya, and H. Harashima. Mechanism of improved gene transfer by the N-terminal stearylation of octaarginine: enhanced cellular association by hydrophobic core formation. Gene Ther. 11:636–644 (2004).
D. Soundara Manickam, H. S. Bisht, L. Wan, G. Mao, and D. Oupicky. Influence of TAT-peptide polymerization on properties and transfection activity of TAT/DNA polyplexes. J. Control. Release 102:293–306 (2005).
Z. Liu, M. Li, D. Cui, and J. Fei. Macro-branched cell-penetrating peptide design for gene delivery. J. Control. Release 102:699–710 (2005).
C. Rudolph, C. Plank, J. Lausier, U. Schillinger, R. H. Muller, and J. Rosenecker. Oligomers of the arginine-rich motif of the HIV-1 TAT protein are capable of transferring plasmid DNA into cells. J. Biol. Chem. 278:11411–11418 (2003).
C. H. Tung, S. Mueller, and R. Weissleder. Novel branching membrane translocational peptide as gene delivery vector. Bioorg. Med. Chem. 10:3609–3614 (2002).
E. Kleemann, M. Neu, N. Jekel, L. Fink, T. Schmehl, T. Gessler, W. Seeger, and T. Kissel. Nano-carriers for DNA delivery to the lung based upon a TAT-derived peptide covalently coupled to PEG-PEI. J. Control. Release 109:299–316 (2005).
K. Kilk, S. El-Andaloussi, P. Jarver, A. Meikas, A. Valkna, T. Bartfai, P. Kogerman, M. Metsis, and U. Langel. Evaluation of transportan 10 in PEI mediated plasmid delivery assay. J. Control. Release 103:511–523 (2005).
J. Zielinski, K. Kilk, T. Peritz, T. Kannanayakal, K. Y. Miyashiro, E. Eiriksdottir, J. Jochems, U. Langel, and J. Eberwine. In vivo identification of ribonucleoprotein-RNA interactions. Proc. Natl. Acad. Sci. U. S. A. 103:1557–1562 (2006).
W. J. Kim, L. V. Christensen, S. Jo, J. W. Yockman, J. H. Jeong, Y. H. Kim, and S. W. Kim. Cholesteryl oligoarginine delivering vascular endothelial growth factor siRNA effectively inhibits tumor growth in colon adenocarcinoma. Mol. Ther. 14:343–350 (2006).
A. Muratovska and M. R. Eccles. Conjugate for efficient delivery of short interfering RNA (siRNA) into mammalian cells. FEBS Lett. 558:63–68 (2004).
M. Zhao, M. F. Kircher, L. Josephson, and R. Weissleder. Differential conjugation of tat peptide to superparamagnetic nanoparticles and its effect on cellular uptake. Bioconjug. Chem. 13:840–844 (2002).
C. Zhang, N. Tang, X. Liu, W. Liang, W. Xu, and V. P. Torchilin. siRNA-containing liposomes modified with polyarginine effectively silence the targeted gene. J. Control. Release 112:229–239 (2006).
R. M. Sawant, J. P. Hurley, S. Salmaso, A. Kale, E. Tolcheva, T. S. Levchenko, and V. P. Torchilin. “SMART” drug delivery systems: double-targeted pH-responsive pharmaceutical nanocarriers. Bioconjug. Chem. 17:943–949 (2006).
J. M. de la Fuente and C. C. Berry. Tat peptide as an efficient molecule to translocate gold nanoparticles into the cell nucleus. Bioconjug. Chem. 16:1176–1180 (2005).
O. A. Garden, P. R. Reynolds, J. Yates, D. J. Larkman, F. M. Marelli-Berg, D. O. Haskard, A. D. Edwards, and A. J. George. A rapid method for labelling CD4+ T cells with ultrasmall paramagnetic iron oxide nanoparticles for magnetic resonance imaging that preserves proliferative, regulatory and migratory behaviour in vitro. J. Immunol. Methods 314:123–133 (2006).
L. Hirt, J. Badaut, J. Thevenet, C. Granziera, L. Regli, F. Maurer, C. Bonny, and J. Bogousslavsky. D-JNKI1, a cell-penetrating c-Jun-N-terminal kinase inhibitor, protects against cell death in severe cerebral ischemia. Stroke 35:1738–1743 (2004).
M. Aarts, Y. Liu, L. Liu, S. Besshoh, M. Arundine, J. W. Gurd, Y. T. Wang, M. W. Salter, M. Tymianski. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science 298:846–850 (2002).
G. Cao, W. Pei, H. Ge, Q. Liang, Y. Luo, F. R. Sharp, A. Lu, R. Ran, S. H. Graham, and J. Chen. In Vivo Delivery of a Bcl-xL Fusion Protein Containing the TAT Protein Transduction Domain Protects against Ischemic Brain Injury and Neuronal Apoptosis. J. Neurosci. 22:5423–5431 (2002).
S. Myou, A. R. Leff, S. Myo, E. Boetticher, J. Tong, A. Y. Meliton, J. Liu, N. M. Munoz, and X. Zhu. Blockade of inflammation and airway hyperresponsiveness in immune-sensitized mice by dominant-negative phosphoinositide 3-kinase-TAT. J. Exp. Med. 198:1573–1582 (2003).
J. C. Clohisy, B. C. Roy, C. Biondo, E. Frazier, D. Willis, S. L. Teitelbaum, and Y. Abu-Amer. Direct inhibition of NF-kappa B blocks bone erosion associated with inflammatory arthritis. J. Immunol. 171:5547–5553 (2003).
E. L. Snyder, B. R. Meade, C. C. Saenz, and S. F. Dowdy. Treatment of terminal peritoneal carcinomatosis by a transducible p53-activating peptide. PLoS. Biol. 2:E36 (2004).
S. Fulda, W. Wick, M. Weller, and K. M. Debatin. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat. Med. 8:808–815 (2002).
L. Yang, T. Mashima, S. Sato, M. Mochizuki, H. Sakamoto, T. Yamori, T. Oh-Hara, and T. Tsuruo. Predominant suppression of apoptosome by inhibitor of apoptosis protein in non-small cell lung cancer H460 cells: therapeutic effect of a novel polyarginine-conjugated Smac peptide. Cancer Res. 63:831–837 (2003).
T. Jiang, E. S. Olson, Q. T. Nguyen, M. Roy, P. A. Jennings, and R. Y. Tsien. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc. Natl. Acad. Sci. U. S. A. 101:17867–17872 (2004).
M. Inoue, M. Mukai, Y. Hamanaka, M. Tatsuta, M. Hiraoka, and S. Kizaka-Kondoh. Targeting hypoxic cancer cells with a protein prodrug is effective in experimental malignant ascites. Int. J. Oncol. 25:713–720 (2004).
J. L. Goldstein, M. S. Brown, R. G. Anderson, D. W. Russell, and W. J. Schneider. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu. Rev. Cell. Biol. 1:1–39 (1985).
V. P. Torchilin, T. S. Levchenko, R. Rammohan, N. Volodina, B. Papahadjopoulos-Sternberg, and G. G. D’Souza. Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome-DNA complexes. Proc. Natl. Acad. Sci. U. S. A. 100:1972–1977 (2003).
C. Rousselle, M. Smirnova, P. Clair, J. M. Lefauconnier, A. Chavanieu, B. Calas, J. M. Scherrmann, and J. Temsamani. Enhanced delivery of doxorubicin into the brain via a peptide-vector-mediated strategy: saturation kinetics and specificity. J. Pharmacol. Exp. Ther. 296:124–131 (2001).
J. R. Maiolo, M. Ferrer, and E. A. Ottinger. Effects of cargo molecules on the cellular uptake of arginine-rich cell-penetrating peptides. Biochim. Biophys. Acta. 1712:161–172 (2005).
Acknowledgement
This work was supported in part by NIH Grants: GM070777 and HL64365.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, L.N., Zaro, J.L. & Shen, WC. Cell Penetrating Peptides: Intracellular Pathways and Pharmaceutical Perspectives. Pharm Res 24, 1977–1992 (2007). https://doi.org/10.1007/s11095-007-9303-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9303-7