Abstract
Purpose
A prerequisite for geldanamycin (GA, NSC122750) to targeting heat shock protein 90 and inhibiting tumor growth is sufficient intracellular drug accumulation. We hypothesized that membrane transporters on tumor cells determine at least in part the response to GA analogues.
Materials and Methods
To facilitate a systematic study of chemosensitivity across a group of GA analogues with similar chemical structures, we correlated mRNA expression profiles of most known transporters with growth inhibitory potencies of compounds in 60 tumor cell lines (NCI-60). We subsequently validated the gene-drug correlations using cytotoxicity and transport assays.
Results
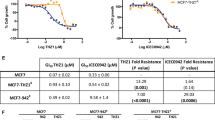
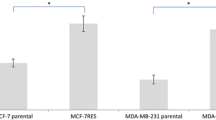
Geldanamycin analogues displayed a range of negative correlations coefficients with ABCB1 (MDR1, or P-glycoprotein) expression. Suppressing ABCB1 in multidrug resistant cells (NCI/ADR-RES and K562/DOX) and ABCB1-transfected cells (BC19) increased sensitivity to GA analogues, as expected for substrates. Moreover, ABCB1-mediated efflux of daunorubicin in K562/DOX cells could be blocked markedly by GA analogues in a dose-dependent fashion. The IC50 values (half-maximum inhibition of daunorubicin efflux) were 5.5, 7.3 and 12 μM for macbecin II (NSC330500), 17-AAG (NSC330507) and GA, respectively.
Conclusions
These observations demonstrate that GA analogues are substrates as well as inhibitors of ABCB1, suggesting that drug interactions between GA analogues and other agents that are ABCB1 substrates may occur via ABCB1 in normal or tumor cells.



Similar content being viewed by others
Abbreviations
- NCI:
-
The National Cancer Institute
- DTP:
-
Developmental Therapeutics Program
- SiRNA:
-
small interfering RNA
- ABC:
-
ATP-binding cassette
- SRB:
-
sulforhodamine B
- GA:
-
geldanamycin
- Hsp90:
-
heat shock protein 90
References
Y. Huang and W. Sadee. Drug sensitivity and resistance genes in cancer chemotherapy: a chemogenomics approach. Drug. Discov. Today 8:356–363 (2003).
U. Scherf, D. T. Ross, M. Waltham, L. H. Smith, J. K. Lee, L. Tanabe, K. W. Kohn, W. C. Reinhold, T. G. Myers, D. T. Andrews, D. A. Scudiero, M. B. Eisen, E. A. Sausville, Y. Pommier, D. Botstein, P. O. Brown, and J. N. Weinstein. A gene expression database for the molecular pharmacology of cancer. Nat. Genet. 24:236–344 (2000).
Y. Huang, P. Anderle, K. J. Bussey, C. Barbacioru, U. Shankavaram, Z. Dai, W. C. Reinhold, A. Papp, J. N. Weinstein, and W. Sadee. Membrane transporters and channels: role of the transportome in cancer chemosensitivity and chemoresistance. Cancer Res. 64:4294–4301 (2004).
Y. Huang, P. E. Blower, C. Yang, C. Barbacioru, Z. Dai, Y. Zhang, J. J. Xiao, K. K. Chan, and W. Sadee. Correlating gene expression with chemical scaffolds of cytotoxic agents: ellipticines as substrates and inhibitors of MDR1. Pharmacogenomics J. 5:112–125 (2005).
L. Whitesell, E. G. Mimnaugh, B. De Costa, C. E. Myers, and L. M. Neckers. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc. Natl. Acad. Sci. U. S. A. 91:8324–8328 (1994).
V. Smith, E. A. Sausville, R. F. Camalier, H. H. Fiebig, and A. M. Burger. Comparison of 17-dimethylaminoethylamino-17-demethoxy-geldanamycin (17DMAG) and 17-allylamino-17-demethoxygeldanamycin (17AAG) in vitro: effects on Hsp90 and client proteins in melanoma models. Cancer Chemother. Pharmacol. 56:126–137 (2005).
G. Chiosis, H. Huezo, N. Rosen, E. Mimnaugh, L. Whitesell, and L. Neckers. 17AAG: low target binding affinity and potent cell activity—finding an explanation. Mol. Cancer Ther. 2:123–129 (2003).
S. Scala, N. Akhmed, U. S. Rao, K. Paull, L. B. Lan, B. Dickstein, J. S. Lee, G. H. Elgemeie, W. D. Stein, and S. E. Bates. P-glycoprotein substrates and antagonists cluster into two distinct groups. Mol. Pharmacol. 51:1024– 1033 (1997).
A. Monks, D. A. Scudiero, G. S. Johnson, K. D. Paull, and E. A. Sausville. The NCI anti-cancer drug screen: a smart screen to identify effectors of novel targets. Anticancer Drug Des. 12:533–541 (1997).
J. N. Weinstein, T. G. Myers, P. M. O’Connor, S. H. Friend, A. J. Fornace, Jr., K. W. Kohn, T. Fojo, S. E. Bates, L. V. Rubinstein, N. L. Anderson, J. K. Buolamwini, W. W. van Osdol, A. P. Monks, D. A. Scudiero, E. A. Sausville, D. W. Zaharevitz, B. Bunow, V. N. Viswanadhan, G. S. Johnson, R. E. Wittes, and K. D. Paull. An information-intensive approach to the molecular pharmacology of cancer. Science 275:343–349 (1997).
B. Efronand and R. Tibshirani. An introduction to the bootstrap, Chapman & Hall, London, 1993.
J. P. Marie, A. M. Faussat-Suberville, D. Zhou, and R. Zittoun. Daunorubicin uptake by leukemic cells: correlations with treatment outcome and mdr1 expression. Leukemia 7:825–831 (1993).
P. Skehan, R. Storeng, D. Scudiero, A. Monks, J. McMahon, D. Vistica, J. T. Warren, H. Bokesch, S. Kenney, and M. R. Boyd. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 82:1107–1112 (1990).
T. L. Riss and R. A. Moravec. Use of multiple assay endpoints to investigate the effects of incubation time, dose of toxin, and plating density in cell-based cytotoxicity assays. Assay Drug Dev. Technol. 2:51–62 (2004).
Y. Huang, Z. Dai, C. Barbacioru, and W. Sadee. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance. Cancer Res. 65:7446–7454 (2005).
E. J. Wang, C. N. Casciano, R. P. Clement, and W. W. Johnson. In vitro flow cytometry method to quantitatively assess inhibitors of P-glycoprotein. Drug Metab. Dispos. 28:522–528 (2000).
Z. Q. Tian, Y. Liu, D. Zhang, Z. Wang, S. D. Dong, C. W. Carreras, Y. Zhou, G. Rastelli, D. V. Santi, and D. C. Myles. Synthesis and biological activities of novel 17-aminogeldanamycin derivatives. Bioorg. Med. Chem. 12:5317–5329 (2004).
D. Li and J. L. Au. Mdr1 transfection causes enhanced apoptosis by paclitaxel: an effect independent of drug efflux function of P-glycoprotein. Pharm. Res. 18:907–913 (2001).
J. Baselga and C. L. Arteaga. Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J. Clin. Oncol. 23:2445–2459 (2005).
Z. Dai, H. Huang, W. Sadee, and P. E. Blower. Chemoinformatics analysis identifies cytotoxic compounds susceptible to chemoresistance mediated by glutathione and cystine/glutamate transport system. J. Med. Chem. 50:1896–1906 (2007).
S. da Rocha Dias, F. Friedlos, Y. Light, C. Springer, P. Workman, and R. Marais. Activated B-RAF is an Hsp90 client protein that is targeted by the anticancer drug 17-allylamino-17-demethoxygeldanamycin. Cancer Res. 65:10686–10691 (2005).
Z. N. Demidenko, W. G. An, J. T. Lee, L. Y. Romanova, J. A. McCubrey, and M. V. Blagosklonny. Kinase-addiction and bi-phasic sensitivity-resistance of Bcr-Abl-and Raf-1-expressing cells to imatinib and geldanamycin. Cancer Biol. Ther. 4:484–490 (2005).
F. Guo, K. Rocha, P. Bali, M. Pranpat, W. Fiskus, S. Boyapalle, S. Kumaraswamy, M. Balasis, B. Greedy, E. S. Armitage, N. Lawrence, and K. Bhalla. Abrogation of heat shock protein 70 induction as a strategy to increase antileukemia activity of heat shock protein 90 inhibitor 17-allylamino-demethoxy geldanamycin. Cancer Res. 65:10536–10544 (2005).
A. Maloney, P. A. Clarke, and P. Workman. Genes and proteins governing the cellular sensitivity to HSP90 inhibitors: a mechanistic perspective. Curr. Cancer Drug Targets 3:331–341 (2003).
L. R. Kell and, S. Y. Sharp, P. M. Rogers, T. G. Myers, and P. Workman. DT-Diaphorase expression and tumor cell sensitivity to 17-allylamino, 17-demethoxygeldanamycin, an inhibitor of heat shock protein 90. J. Natl. Cancer Inst. 91:1940–1949 (1999).
Y. Huang and W. Sadee. Membrane transporters and channels in chemoresistance and -sensitivity of tumor cells. Cancer Lett. 239:168–182 (2006).
K. V. Chin, I. Pastan, and M. M. Gottesman. Function and regulation of the human multidrug resistance gene. Adv. Cancer Res. 60:157–180 (1993).
M. M. Gottesman and I. Pastan. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu. Rev. Biochem. 62:385–427 (1993).
R. Perez-Tomas. Multidrug resistance: retrospect and prospects in anti-cancer drug treatment. Curr. Med. Chem. 13:1859–1876 (2006).
P. Anderle, Y. Huang, and W. Sadee. Intestinal membrane transport of drugs and nutrients: genomics of membrane transporters using expression microarrays. Eur. J. Pharm. Sci. 21:17–24 (2004).
M. J. Egorin, D. M. Rosen, J. H. Wolff, P. S. Callery, S. M. Musser, and J. L. Eiseman. Metabolism of 17-(allylamino)-17-demethoxygeldanamycin (NSC 330507) by murine and human hepatic preparations. Cancer Res. 58:2385–2396 (1998).
V. J. Wacher, C. Y. Wu, and L. Z. Benet. Overlapping substrate specificities and tissue distribution of cytochrome P450 3A and P-glycoprotein: implications for drug delivery and activity in cancer chemotherapy. Mol. Carcinog. 13:129–134 (1995).
S. Kajiji, J. A. Dreslin, K. Grizzuti, and P. Gros. Structurally distinct MDR modulators show specific patterns of reversal against P-glycoproteins bearing unique mutations at serine939/941. Biochemistry 33:5041–5048 (1994).
A. Hamada, H. Miyano, H. Watanabe, and H. Saito. Interaction of imatinib mesilate with human P-glycoprotein. J. Pharmacol. Exp. Ther. 307:824–828 (2003).
M. P. Goetz, D. Toft, J. Reid, M. Ames, B. Stensgard, S. Safgren, A. A. Adjei, J. Sloan, P. Atherton, V. Vasile, S. Salazaar, A. Adjei, G. Croghan, and C. Erlichman. Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. J. Clin. Oncol. 23:1078–1087 (2005).
Acknowledgments
We thank the staff of NCI DTP for generation of the pharmacological database used in this study. This work was supported by NIH grant GM61390 and by the Food and Drug Administration.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, Y., Blower, P.E., Liu, R. et al. Chemogenomic Analysis Identifies Geldanamycins as Substrates and Inhibitors of ABCB1. Pharm Res 24, 1702–1712 (2007). https://doi.org/10.1007/s11095-007-9300-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9300-x