Abstract
Purpose
It was the aim of this study to identify the governing mechanisms during protein release from cylindrical lipid matrices by visualizing mass transport and correlating the data with in vitro dissolution testing.
Materials and Methods
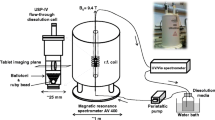
Glyceryl trimyristate cylinders of 2 mm diameter, 2.2 mm height and 7 mg weight were manufactured by compression of a protein–lipid powder mixture prepared by a polyethylene glycol (PEG) co-lyophilization technique. BSA was fluorescence-labeled and the distribution visualized and quantified at different stages of the release process by confocal microscopy in parallel to the quantification in the release buffer. The impact of matrix loading and protein molecular weight was assessed with the model proteins lysozyme, BSA, alcohol dehydrogenase and thyroglobulin.
Results
Buffer penetration and protein release occurred simultaneously from the outer regions of the cylinder progressing towards the center. Release from the top and bottom of the matrix was not negligible but much slower than penetration from the side, probably due to an oriented arrangement of lipid flakes during compression. The different quantification strategies were found to yield identical results. At 6% protein loading, buffer penetration was complete after 4 days, while only 60% of the protein was liberated in that time and release continued up to day 63. Protein release kinetics could be described using the power law equation M t /M ∞ = kt n with an average time exponent n of 0.45 (±0.04) for loadings varying between 1 and 8%. A percolation threshold at 5% pure protein loading and 3–4% mixed loading (PEG and protein at a 1:1 mass ratio) could be identified. Release rate was found to decrease with increasing molecular weight.
Conclusions
Protein release from lipid-based matrices is a purely diffusion controlled mechanism. Potential protein stabilization approaches should address the time span between complete buffer penetration of the matrix and 100% release of the remaining loading, which would be exposed to an aqueous environment before leaving the matrix.










Similar content being viewed by others
Abbreviations
- ADH:
-
alcohol dehydrogenase
- BCA:
-
biscinchoninic acid
- BSA:
-
bovine serum albumin
- CLSM:
-
confocal laser scanning microscopy
- DCM:
-
dichloromethane
- DIPEA:
-
N-ethyldiisopropylamine
- DMSO:
-
dimethylsulfoxide
- FITC:
-
fluorescein isothiocyanate
- IL:
-
interleukin
- mPEG-NH2 :
-
methoxy-PEG-amine
- MWCO:
-
molecular weight cut-off
- PEG:
-
polyethylene glycol
- TAMRA:
-
carboxy-tetramethylrhodamine
- SRH:
-
sulforhodamine 101 hydrate
- THF:
-
tetrahydrofurane
References
V. R. Sinha, and A. Trehan. Biodegradable microspheres for protein delivery. J. Control Release 90(3):261–280 (2003).
C. Guse, S. Koennings, A. Maschke, M. Hacker, C. Becker, S. Schreiner, T. Blunk, T. Spruss, and A. Goepferich. Biocompatibility and erosion behavior of implants made of triglycerides and blends with cholesterol and phospholipids. Int. J. Pharm. 314(2):153–160 (2006).
S. Koennings, A. Sapin, T. Blunk, P. Menei, and A. Göpferich. Towards controlled release of BDNF-Manufacturing strategies for protein-loaded lipid implants and biocompatibility evaluation in the brain. J. Control Release, in press (2007).
W. Vogelhuber, E. Magni, A. Gazzaniga, and A. Goepferich. Monolithic glyceryl trimyristate matrices for parenteral drug release applications. Eur. J. Pharm. Biopharm. 55(1):133–138 (2003).
B. Appel, A. Maschke, B. Weiser, H. Sarhan, C. Englert, P. Angele, T. Blunk, and A. Gopferich. Lipidic implants for controlled release of bioactive insulin: effects on cartilage engineered in vitro. Int. J. Pharm. 314(2):170–178 (2006).
A. Maschke, C. Becker, D. Eyrich, J. Kiermaier, T. Blunk, and A. Gopferich, Development of a spray congealing process for the preparation of insulin-loaded lipid microparticles and characterization thereof. Eur. J. Pharm. Biopharm. 65(2):175–187 (2007).
H. Reithmeier, J. Herrmann, and A. Gopferich. Lipid microparticles as a parenteral controlled release device for peptides. J. Control Release 73(2–3):339–350 (2001).
H. Reithmeier, J. Herrmann, and A. Gopferich. Development and characterization of lipid microparticles as a drug carrier for somatostatin. Int. J. Pharm. 218(1–2):133–143 (2001).
S. Mohl, and G. Winter. Continuous release of rh-interferon á-2a from triglyceride matrices. J. Control Release 97(1):67–78 (2004).
W. Vogelhuber, E. Magni, M. Mouro, T. Spruss, C. Guse, A. Gazzaniga, and A. Gopferich. Monolithic triglyceride matrices: a controlled-release system for proteins. Pharm. Dev. Technol. 8(1):71–79 (2003).
S. Koennings, E. Garcion, N. Faisant, P. Menei, J. P. Benoit, and A. Goepferich. In vitro investigation of lipid implants as a controlled release system for interleukin-18. Int. J. Pharm. 314(2):145–152 (2006).
A. Shenderova, T. G. Burke, and S. P. Schwendeman. Evidence for an acidic microclimate in PLGA microspheres. In Proceedings of the International Symposium on Controlled Release of Bioactive Materials, 2001, vol. 25 pp. 265–266.
T. Estey, J. Kang, S. P. Schwendeman, and J. F. Carpenter. BSA degradation under acidic conditions: a model for protein instability during release from PLGA delivery systems. J. Pharm. Sci. 95(7):1626–1639 (2006).
G. Zhu, and S. P. Schwendeman. Stabilization of proteins encapsulated in cylindrical poly(lactide-co-glycolide) implants: mechanism of stabilization by basic additives. Pharm. Res. 17(3):351–357 (2000).
A. Lucke, J. Kiermaier and A. Gopferich. Peptide acylation by poly(alpha-hydroxy esters). Pharm. Res. 19(2):175–181 (2001).
C. Guse, S. Koennings, F. Kreye, F. Siepmann, A. Goepferich, and J. Siepmann. Drug release from lipid-based implants: elucidation of the underlying mass transport mechanisms. Int. J. Pharm. 314(2):137–144 (2006).
A. Messaritaki, S. J. Black, C. F. van der Walle, and S. P. Rigby. NMR and confocal microscopy studies of the mechanisms of burst drug release from PLGA microspheres. J. Control Release 108(2–3):271–281 (2005).
P. K. Smith, R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson, and D. C. Klenk. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76–85 (1985).
C. Guermant, J. Brygier, D. Baeyens-Volant, M. Nijs, J. Vincentelli, C. Paul, and Y. Looze. Quantitative determination of polyethylene glycol based upon its salting out and partitioning of a dye into the resulting aqueous two-phase system. Anal. Biochem. 230(2):254–258 (1995).
P. L. Ritger, and N. A. Peppas. A simple equation for description of solute release: I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control Release. 5(1):23–36 (1987).
M. Le Maire, A. Ghazi, J. V. Moeller, and L. P. Aggerbeck. The use of gel chromatography for the determination of sizes and relative molecular masses of proteins. Interpretation of calibration curves in terms of gel-pore-size distribution. Biochem. J. 243(2):399–404 (1987).
F. Carli, and L. Simioni. Kinetics of liquid capillary penetration into inert polymer matrixes. Pharm. Acta Helv. 53(11):320–326 (1978).
R. Collins. Mathematical modelling of controlled release from implanted drug-impregnated monoliths. Pharm. Sci. Technol. Today 1(6):269–276 (1998).
P. Colombo, R. Bettini, P. L. Catellani, P. Santi, and N. A. Peppas. Drug volume fraction profile in the gel phase and drug release kinetics in hydroxypropyl methyl cellulose matrixes containing a soluble drug. Eur. J. Pharm. Biopharm. 9(1):33–40 (1999).
S. W. Sun, Y. I. Jeong, S. W. Jung, and S. H. Kim. Characterization of FITC-albumin encapsulated poly(DL-lactide-co-glycolide) microspheres and its release characteristics. J. Microencapsul. 20(4):479–488 (2003).
O. Hosoya, S. Chono, Y. Sako, K. Juni, K. Morimoto, and T. Seki. Determination of diffusion coefficients of peptides and prediction of permeability through a porous membrane. J. Pharm. Pharmacol. 56(129):1501–1507 (2004).
D. Shugar. Measurement of lysozyme activity and the ultraviolet inactivation of lysozyme. Biochim. Biophys. Acta 8:302–309 (1952).
P. Jolles, and J. Jolles. What’s new in lysozyme research? Always a model system, today as yesterday. Mol. Cell. Biochem. 63(2):165–189 (2001).
R. A. Siegel, J. Kost, and R. Langer. Mechanistic studies of macromolecular drug release from macroporous polymers. I. Experiments and preliminary theory concerning completeness of drug release. J. Control Release 8(3):223–236 (1989).
J. D. Bonny, and H. Leuenberger. Matrix type controlled release systems: I. Effect of percolation on drug dissolution kinetics. Pharm. Acta Helv. 66(5–6):160–164 (1991).
J. D. Bonny, and H. Leuenberger. Matrix type controlled release systems II. Percolation effects in non-swellable matrices. Pharm. Acta Helv. 68(1):25–33 (1993).
I. Caraballo, M. Millán, and A. M. Rabasco. Relationship between drug percolation threshold and particle size in matrix tablets. Pharm. Res. 13(3):387–390 (1996).
M. Millán, I. Caraballo, and A. M. Rabasco. The role of the drug/excipient particle size ratio in the percolation model for tablets. Pharm. Res. 15(2):216–220 (1998).
Acknowledgements
The authors are grateful for the support of this work by the European Commission (Research and Technological Development Project; BCDDS: Biodegradable Controlled Drug Delivery Systems for the Treatment of Brain Diseases; Contract No.QLK3-CT-2001-02226).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koennings, S., Tessmar, J., Blunk, T. et al. Confocal Microscopy for the Elucidation of Mass Transport Mechanisms Involved in Protein Release from Lipid-based Matrices. Pharm Res 24, 1325–1335 (2007). https://doi.org/10.1007/s11095-007-9258-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9258-8