Abstract
Purpose
The purpose of this paper was to identify the location of a succinimide and determine the rate of its formation and hydrolysis in a recombinant human monoclonal IgG2 antibody aged in mildly acidic buffers at elevated temperatures.
Materials and Methods
Cation exchange (CEX) HPLC separated multiple Main Peaks and high levels (up to 50%) of basic variants, the identification of which was an analytical challenge and required several complementary techniques. The relative abundance of the CEX basic variants was used to quantify the percentage of succinimide and to study the rates of its formation and hydrolysis.
Results
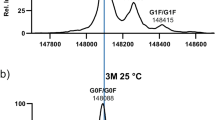
Mass decrease by approximately 18 Da for intact antibodies from the CEX basic fractions suggested succinimide formation from aspartic acid as the major modification. Reversed-phase HPLC/MS of the reduced and trypsin-digested samples detected an isoaspartate 30 (isoD30) in the light chain peptide A25-R37. Direct evidence that isoD30 was from succinimide was obtained by performing succinimide hydrolysis in \( {\text{H}}_{{\text{2}}} {}^{{{\text{18}}}}{\text{O}}\) followed by tryptic digestion in \( {\text{H}}_{{\text{2}}} {}^{{{\text{16}}}}{\text{O}}\).
Conclusions
Succinimide formation increased as pH became more acidic, whereas its hydrolysis was faster as pH became neutral and alkaline. Succinimide hydrolysis in a denatured sample was estimated to have completed in less than 2 h, but approximately three days for a similar pH but without denaturant. These observations suggest that protein conformation affects succinimide hydrolysis.










Similar content being viewed by others
References
A. C. Herman, T. C. Boone, and H. S. Lu. Characterization, formulation, and stability of Neupogen (Filgrastim), a recombinant human granulocyte-colony stimulating factor. In R. Pearlman and Y. J. Wang (eds.), Formulation, Characterization, and Stability of Protein Drugs. Case Histories, Plenum, New York, 1996, pp. 303–328.
X. M. Lam, J. Y. Yang, and J. L. Cleland. Antioxidants for prevention of methionine oxidation in recombinant monoclonal antibody HER2. J. Pharm. Sci. 86:1250–1255 (1997).
S. A. Bernhard, A. Berger, J. H. Carter, E. Katchalski, M. Sela, and Y. Shalitin. Co-operative effects of functional groups in peptides. I. Aspartyl-serine derivatives. J. Am. Chem. Soc. 84:2421–2434 (1962).
T. Geiger and S. Clarke. Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. J. Biol. Chem. 262:785–794 (1987).
J. Cacia, R. Keck, L. G. Presta, and J. Frenz. Isomerization of an aspartic acid residue in the complementarity-determining regions of a recombinant antibody to human IgE: identification and effect on binding affinity. Biochemistry 35:1897–1903 (1996).
R. J. Harris, B. Kabakoff, F. D. Macchi, F. J. Shen, M. Y. Kwong, J. D. Andya, S. J. Shire, N. Bjork, K. Totpal, and A. B. Chen. Identification of multiple sources of charge heterogeneity in a recombinant antibody. J. Chromatogr., B, Biomed. Sci. Appl. 752:233–245 (2001).
H. A. Doyle, R. J. Gee, and M. J. Mamula. A failure to repair self-proteins leads to T cell hyperproliferation and autoantibody production. J. Immunol. 171:2840–2847 (2003).
H. A. Doyle, J. Zhou, M. J. Wolff, B. P. Harvey, R. M. Roman, R. J. Gee, R. A. Koski, and M. J. Mamula. Isoaspartyl posttranslational modification triggers anti-tumor T and B lymphocyte immunity. J. Biol. Chem. 281:32676–32683 (2006).
M. L. Yang, H. A. Doyle, R. J. Gee, J. D. Lowenson, S. Clarke, B. R. Lawson, D. W. Aswad, and M. J. Mamula. Intracellular protein modification associated with altered T cell functions in autoimmunity. J. Immunol. 177:4541–4549 (2006).
W. Zhang and M. J. Czupryn. Analysis of isoaspartate in a recombinant monoclonal antibody and its charge isoforms. J. Pharm. Biomed. Anal. 30:1479–1490 (2003).
S. Capasso, A. J. Kirby, S. Salvadori, F. Sica, and A. Zagari. Kinetics and mechanism of the reversible isomerization of aspartic acid residues in tetrapeptides. J. Chem. Soc., Perkin Trans., II 437–442 (1995).
C. Oliyai and R. T. Borchardt. Chemical pathways of peptide degradation. IV. Pathways, kinetics, and mechanism of degradation of an aspartyl residue in a model hexapeptide. Pharm. Res. 10:95–102 (1993).
C. Oliyai and R. T. Borchardt. Solution and solid-state chemical instabilities of asparaginyl and aspartyl residues in model peptides. In J. L. Cleland and R. Langer (eds.), Formulation and Delivery for Proteins and Peptides, American Chemical Society, Washington, DC, 1994, pp. 47–58.
G. Teshima, J. T. Stults, V. Ling, and E. Canova-Davis. Isolation and characterization of a succinimide variant of methionyl human growth hormone. J. Biol. Chem. 266:13544–13547 (1991).
Y. Sadakane, T. Yamazaki, K. Nakagomi, T. Akizawa, N. Fujii, T. Tanimura, M. Kaneda, and Y. Hatanaka. Quantification of the isomerization of Asp residue in recombinant human alpha A-crystallin by reversed-phase HPLC. J. Pharm. Biomed. Anal. 30:1825–1833 (2003).
C. Milstein and J. R. Pink. Structure and evolution of immunoglobulins (review). Prog. Biophys. Mol. Biol. 21:209–263 (1970).
H. Tomizawa, H. Yamada, T. Ueda, and T. Imoto. Isolation and characterization of 101-succinimide lysozyme that possesses the cyclic imide at Asp101-Gly102. Biochemistry 33:8770–8774 (1994).
H. Tomizawa, H. Yamada, Y. Hashimoto, and T. Imoto. Stabilization of lysozyme against irreversible inactivation by alterations of the Asp-Gly sequences. Protein Eng. 8:1023–1028 (1995).
J. Najbauer, J. Orpiszewski, and D. W. Aswad. Molecular aging of tubulin: accumulation of isoaspartyl sites in vitro and in vivo. Biochemistry 35:5183–5190 (1996).
D. W. Aswad, M. V. Paranandi, and B. T. Schurter. Isoaspartate in peptides and proteins: formation, significance, and analysis. J. Pharm. Biomed. Anal. 21:1129–1136 (2000).
R. Bischoff, P. Lepage, M. Jaquinod, G. Cauet, M. Acker-Klein, D. Clesse, M. Laporte, A. Bayol, A. van Dorsselaer, and C. Roitsch. Sequence-specific deamidation: isolation and biochemical characterization of succinimide intermediates of recombinant hirudin. Biochemistry 32:725–734 (1993).
R. C. Stephenson and S. Clarke. Succinimide formation from aspartyl and asparaginyl peptides as a model for the spontaneous degradation of proteins. J. Biol. Chem. 264:6164–6170 (1989).
J. B. Stimmel, B. M. Merrill, L. F. Kuyper, C. P. Moxham, J. T. Hutchins, M. E. Fling, and F. C. Kull, Jr. Site-specific conjugation on serine right-arrow cysteine variant monoclonal antibodies. J. Biol. Chem. 275:30445–30450 (2000).
J. A. Lindquist and P. N. McFadden. Incorporation of two 18O atoms into a peptide during isoaspartyl repair reveals repeated passage through a succinimide intermediate. J. Protein Chem. 13:553–560 (1994).
P. Schindler, D. Muller, W. Marki, H. Grossenbacher, and W. J. Richter. Characterization of a beta-Asp33 isoform of recombinant hirudin sequence variant 1 by low-energy collision-induced dissociation. J. Mass Spectrom. 31:967–974 (1996).
T. M. Dillon, P. V. Bondarenko, D. S. Rehder, G. D. Pipes, G. R. Kleemann, and M. S. Ricci. Optimization of a reversed-phase LC/MS method for characterizing recombinant antibody heterogeneity and stability. J. Chromatogr., A 1120:112–120 (2006).
D. S. Rehder, T. M. Dillon, G. D. Pipes, and P. V. Bondarenko. Reversed-phase LC/MS analysis of reduced monoclonal antibodies in pharmaceutics. J. Chromatogr., A 1102:164–175 (2006).
H. S. Gadgil, G. D. Pipes, T. M. Dillon, M. J. Treuheit, and P. V. Bondarenko. Improving mass accuracy of high performance liquid chromatography/electrospray ionization time-of-flight mass spectrometry of monoclonal antibodies. J. Am. Soc. Mass Spectrom. 17:867–872 (2006).
D. Chelius, D. S. Rehder, and P. V. Bondarenko. Identification and characterization of deamidation sites in the conserved regions of human immunoglobulin gamma antibodies. Anal. Chem. 77:6004–6011 (2005).
Z. Zhang. Prediction of low-energy collision-induced dissociation spectra of peptides. Anal. Chem. 76:3908–3922 (2004).
Z. Zhang. De novo peptide sequencing based on a divide-and-conquer algorithm and peptide tandem spectrum simulation. Anal. Chem. 76:6374–6383 (2004).
D. Chelius, K. Jing, A. Lueras, D. S. Rehder, T. M. Dillon, A. Vizel, R. S. Rajan, T. Li, M. J. Treuheit, and P. V. Bondarenko. Formation of pyroglutamic acid from N-terminal glutamic acid in immunoglobulin gamma antibodies. Anal. Chem. 78:2370–2376 (2006).
M. Xie, D. V. Velde, M. Morton, R. T. Borchardt, and R. L. Schowen. pH-Induced change in the rate-determining step for the hydrolysis of the Asp/Asn-derived cyclic-imide intermediate in protein degradation. J. Am. Chem. Soc. 118:8955–8956 (1996).
Acknowledgements
We would like to thank Thomas Dillon for help with the RP HPLC of the intact IgG2 antibody; Alexis Lueras for her assistance with preparation of some of the aged protein samples; Barbara Norwood for her assistance in bioactivity measurements; Tiansheng Li for his support and David Brems for his comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chu, G.C., Chelius, D., Xiao, G. et al. Accumulation of Succinimide in a Recombinant Monoclonal Antibody in Mildly Acidic Buffers Under Elevated Temperatures. Pharm Res 24, 1145–1156 (2007). https://doi.org/10.1007/s11095-007-9241-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9241-4