Abstract
Purpose
We examined the effects of the nine nonsynonymous germ-line mutations/SNPs in the breast cancer resistance protein (BCRP/ABCG2) gene on the expression and function of the protein.
Materials and Methods
We generated cDNAs for each of these mutants (G151T, C458T, C496G, A616C, T623C, T742C, T1291C, A1768T, and G1858A BCRP) and compared the effects of their exogenous expression in PA317 cells with a wild-type control.
Results
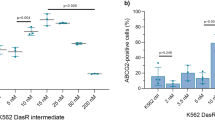
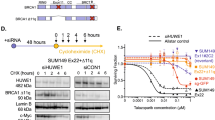
PA/F208S cells (T623C BCRP-transfectants) expressed marginal levels of a BCRP protein species (65kDa), which is slightly smaller than wild-type (70kDa), but this mutant did not appear on the cell surface or confer drug resistance. PA/F431L cells (T1291C BCRP-transfectants) were found to express both 70kDa and 65kDa BCRP protein products. In addition, although PA/F431L cells expressed 70kDa BCRP at comparable levels to PA/WT cells, they showed only marginal resistance to SN-38. PA/T153M cells (C458T BCRP-transfectants) and PA/D620N cells (G1858A BCRP-transfectants) expressed lower amounts of BCRP and showed lower levels of resistance to SN-38 compared with PA/WT cells.
Conclusions
We have shown that T623C BCRP encodes a non-functional BCRP and that T1291C BCRP encodes a low-functional BCRP. Hence, these mutations may affect the pharmacokinetics of BCRP substrates in patients harboring these alleles.




Similar content being viewed by others
Abbreviations
- ABC transporter:
-
ATP-binding cassette transmembrane transporter
- BCRP:
-
breast cancer resistance protein
- DHFR:
-
dihydrofolate reductase
- GAPDH:
-
glyceraldehyde-3-phosphate dehydrogenase
- IRES:
-
internal ribosome entry site
- SNPs:
-
single nucleotide polymorphisms
References
M. M. Gottesman, C. A. Hrycyna, P. V. Schoenlein, U. A. Germann, and I. Pastan. Genetic analysis of the multidrug transporter. Annu. Rev. Genet. 29:607–649 (1995).
C. J. Chen, J. E. Chin, K. Ueda, D. P. Clark, I. Pastan, M. M. Gottesman, and I. B. Roninson. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell 47:381–389 (1986).
S. P. Cole, G. Bhardwaj, J. H. Gerlach, J. E. Mackie, C. E. Grant, K. C. Almquist, A. J. Stewart, E. U. Kurz, A. M. Duncan, and R. G. Deeley. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 258:1650–1654 (1992).
R. Allikmets, L. M. Schriml, A. Hutchinson, V. Romano-Spica, and M. Dean. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 58:5337–5339 (1998).
L. A. Doyle, W. Yang, L. V. Abruzzo, T. Krogmann, Y. Gao, A. K. Rishi, and D. D. Ross. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. U.S.A. 95:15665–15670 (1998).
K. Miyake, L. Mickley, T. Litman, Z. Zhan, R. Robey, B. Cristensen, M. Brangi, L. Greenberger, M. Dean, T. Fojo, and S. E. Beates. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 59:8–13 (1999).
M. Maliepaard, M. A. van Gastelen, L. A. de Jong, D. Pluim, R. C. van Waardenburg, M. C. Ruevekamp-Helmers, B. G. Floot, and J. H. Schellens. Overexpression of the BCRP/MXR/ABCP gene in a topotecan-selected ovarian tumor cell line. Cancer Res. 59:4559–4563 (1999).
S. Kawabata, M. Oka, K. Shiozawa, K. Tsukamoto, K. Nakatomi, H. Soda, M. Fukuda, Y. Ikegami, K. Sugahara, Y. Yamada, S. Kamihira, L. A. Doyle, D. D. Ross, and S. Kohno. Breast cancer resistance protein directly confers SN-38 resistance of lung cancer cells. Biochem. Biophys. Res. Commun. 280:1216–1223 (2001).
K. Kage, S. Tsukahara, T. Sugiyama, S. Asada, E. Ishikawa, T. Tsuruo, and Y. Sugimoto. Dominant-negative inhibition of breast cancer resistance protein as drug efflux pump through the inhibition of S-S dependent homodimerization. Int. J. Cancer 97:626–630 (2002).
K. Kage, T. Fujita, and Y. Sugimoto. Role of Cys-603 in dimer/oligomer formation of the breast cancer resistance protein BCRP/ABCG2. Cancer Sci. 96:866–872 (2005).
Y. Imai, S. Asada, S. Tsukahara, E. Ishikawa, T. Tsuruo, and Y. Sugimoto. Breast cancer resistance protein exports sulfated estrogens but not free estrogens. Mol. Pharmacol. 64:610–618 (2003).
M. Maliepaard, G. L. Scheffer, I. F. Faneyte, M. A. van Gastelen, A. C. Pijnenborg, A. H. Schinkel, M. J. van De Vijver, R. J. Scheper, and J. H. Schellens. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 61:3458–3464 (2001).
S. Zhou, J. D. Schuetz, K. D. Bunting, A. M. Colapietro, J. Sampath, J. J. Morris, I. Lagutinal, G. C. Grosveld, M. Osawa, H. Nakauchi, and B. P. Sorrentino. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med. 7:1028–1034 (2001).
D. M. van der Kolk, E. Vellenga, G. L. Scheffer, M. Muller, S. E. Bates, R. J. Scheper, E. G. de Vries. Expression and activity of breast cancer resistance protein (BCRP) in de novo and relapsed acute myeloid leukemia. Blood 99:3763–3770 (2002).
M. M. van den Heuvel-Eibrink, E. A. Wiemer, A. Prins, J. P. Meijerink, P. J. Vossebeld, B. van der Holt, R. Pieters, P. Sonneveld. Increased expression of the breast cancer resistance protein (BCRP) in relapsed or refractory acute myeloid leukemia (AML). Leukemia 16:833–839 (2002).
J. W. Jonker, G. Merino, S. Musters, A. E. van Herwaarden, E. Bolscher, E. Wagenaar, E. Mesman, T. C. Dale, and A. H. Schinkel. The breast cancer resistance protein BCRP (ABCG2) concentrates drugs and carcinogenic xenotoxins into milk. Nat. Med. 11:127–129 (2005).
Y. Imai, M. Nakane, K. Kage, S. Tsukahara, E. Ishikawa, T. Tsuruo, Y. Miki, and Y. Sugimoto. C421A polymorphism in the human breast cancer resistance protein gene is associated with low expression of Q141K protein and low-level drug resistance. Mol. Cancer Ther. 1:611–616 (2002).
A. Sparreboom, H. Gelderblom, S. Marsh, R. Ahluwalia, R. Obach, P. Principe, C. Twelves, J. Verweij, and H. L. Mcleod. Diflomotecan pharmacokinetics in relation to ABCG2 421C>A genotype. Clin. Pharmacol. Ther. 76:38–44 (2004).
W. Zhang, B. N. Yu, Y. J. He, L. Fan, Q. Li, Z. Q. Liu, A. Wang, Y. L. Liu, Z. R. Tan, Fen-Jiang, Y. F. Huang, H. H. Zhou. Role of BCRP 421C>A polymorphism on rosuvastatin pharmacokinetics in healthy Chinese males. Clin. Chim. Acta 373:99–103 (2006).
Y. Sugimoto, S. Tsukahara, S. Sato, M. Suzuki, H. Nunoi, H. L. Malech, M. M. Gottesman, and T. Tsuruo. Drug-selected co-expression of P-glycoprotein and gp91 in vivo from an MDR1-bicistronic retrovirus vector Ha-MDR-IRES-gp91. J. Gene Med. 5:366–376 (2003).
Y. Sugimoto, I. Aksentijevich, M. M. Gottesman, and I. Pastan. Efficient expression of drug-selectable genes in retroviral vectors under control of an internal ribosome entry site. Biotechnology (N Y) 12:694–698 (1994).
Y. Sugimoto, C. A. Hrycyna, I. I. Aksentijevich, I. I. Pastan, and M. M. Gottesman. Coexpression of a multidrug-resistance gene (MDR1) and herpes simplex virus thymidine kinase gene as part of a bicistronic messenger RNA in a retrovirus vector allows selective killing of MDR1-transduced cells. Clin. Cancer Res. 1:447–457 (1995).
Y. Sugimoto, S. Sato, S. Tsukahara, M. Suzuki, E. Okochi, M. M. Gottesman, I. Pastan, and T. Tsuruo. Coexpression of a multidrug resistance gene (MDR1) and herpes simplex virus thymidine kinase gene in a bicistronic retroviral vector Ha-MDR-IRES-TK allows selective killing of MDR1-transduced human tumors transplanted in nude mice. Cancer Gene Ther. 4:51–58 (1997).
Z. S. Chen, R. W. Robey, M. G. Belinsky, I. Shchaveleva, X. Q. Ren, Y. Sugimoto, D. D. Ross, S. E. Bates, and G. D. Kruh. Transport of methotrexate, methotrexate polyglutamates, and 17beta-estradiol 17-(beta-D-glucuronide) by ABCG2: effects of acquired mutations at R482 on methotrexate transport. Cancer Res. 63:4048–4054 (2003).
E. L. Volk, K. M. Farley, Y. Wu, F. Li, R. W. Robey, and E. Schneider. Overexpression of wild-type breast cancer resistance protein mediates methotrexate resistance. Cancer Res. 62:5035–5040 (2002).
L. F. Payen, M. Gao, C. J. Westlake, S. P. Cole, and R. G. Deeley. Role of carboxylate residues adjacent to the conserved core Walker B motifs in the catalytic cycle of multidrug resistance protein 1 (ABCC1). J. Biol. Chem. 278:38537–38547 (2003).
K. Mutoh, J. Mitsuhashi, Y. Kimura, S. Tsukahara, E. Ishikawa, K. Sai, S. Ozawa, J. Sawada, K. Ueda, K. Katayama, and Y. Sugimoto. A T3587G germ-line mutation of the MDR1 gene encodes a nonfunctional P-glycoprotein. Mol. Cancer Ther. 5:877–884 (2006).
M. Wada, S. Toh, K. Taniguchi, T. Nakamura, T. Uchiumi, K. Kohno, I. Yoshida, A. Kimura, S. Sakisaka, Y. Adachi, and M. Kuwano. Mutations in the canilicular multispecific organic anion transporter (cMOAT) gene, a novel ABC transporter, in patients with hyperbilirubinemia II/Dubin-Johnson syndrome. Hum. Mol. Genet. 7:203–207 (1998).
K. Hashimoto, T. Uchiumi, T. Konno, T. Ebihara, T. Nakamura, M. Wada, S. Sakisaka, F. Maniwa, T. Amachi, K. Ueda, and M. Kuwano. Trafficking and functional defects by mutations of the ATP-binding domains in MRP2 in patients with Dubin-Johnson syndrome. Hepatology 36:1236–1245 (2002).
V. Keitel, J. Kartenbeck, A. T. Nies, H. Spring, M. Brom, and D. Keppler. Impaired protein maturation of the conjugate export pump multidrug resistance protein 2 as a consequence of a deletion mutation in Dubin-Johnson syndrome. Hepatology 32:1317–1328 (2000).
M. Miwa, S. Tsukahara, E. Ishikawa, S. Asada, Y. Imai, and Y. Sugimoto. Single amino acid substitutions in the transmembrane domains of breast cancer resistance protein (BCRP) alter cross resistance patterns in transfectants. Int. J. Cancer 107:757–763 (2003).
S. Mizuarai, N. Aozasa, and H. Kotani. Single nucleotide polymorphisms result in impaired membrane localization and reduced atpase activity in multidrug transporter ABCG2. Int. J. Cancer 109:238–246 (2004).
C. P. Zamber, J. K. Lamba, K. Yasuda, J. Farnum, K. Thummel, J. D. Schuetz, and E. G. Schuetz. Natural allelic variants of breast cancer resistance protein (BCRP) and their relationship to BCRP expression in human intestine. Pharmacogenetics 13:19–28 (2003).
M. Itoda, Y. Saito, K. Shirao, H. Minami, A. Ohtsu, T. Yoshida, N. Saijo, H. Suzuki, Y. Sugiyama, S. Ozawa, and J. Sawada. Eight novel single nucleotide polymorphisms in ABCG2/BCRP in Japanese cancer patients administered irinotacan. Drug Metab. Pharmacokinet. 18:212–217 (2003).
Y. Honjo, K. Morisaki, L. M. Huff, R. W. Robey, J. Hung, M. Dean, and S. E. Bates. Single-nucleotide polymorphism (SNP) analysis in the ABC half-transporter ABCG2 (MXR/BCRP/ABCP1). Cancer Biol. Ther. 1:696–702 (2002).
Acknowledgments
This study was supported by the Ministry of Education, Culture, Sports, Science and Technology, and the Ministry of Health, Labor and Welfare, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshioka, S., Katayama, K., Okawa, C. et al. The Identification of Two Germ-line Mutations in the Human Breast Cancer Resistance Protein Gene that Result in the Expression of a Low/Non-functional Protein. Pharm Res 24, 1108–1117 (2007). https://doi.org/10.1007/s11095-007-9235-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9235-2