Abstract
Purpose
This work examines the cause of aggregation of an Fc-fusion protein formulated in sorbitol upon frozen storage for extended periods of time at −30°C.
Materials and Methods
We designed sub-ambient differential scanning calorimetry (DSC) experiments to capture the effects of long-term frozen storage. The physical stability of formulation samples was monitored by size exclusion high performance liquid chromatography (SE-HPLC).
Results
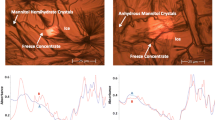
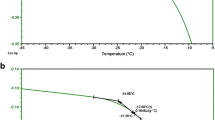
DSC analysis of non-frozen samples shows the expected glass transitions (Tg′) at −45°C for samples in sorbitol and at −32°C in sucrose. In time course studies where sorbitol formulations were stored at −30°C and analyzed by DSC without thawing, two endothermic transitions were observed: a melting endotherm at −20°C dissipated over time, and a second endotherm at −8°C was seen after approximately 2 weeks and persisted in all later time points. Protein aggregation was only seen in the samples formulated in sorbitol and stored at −30°C, correlating aggregation with the aforementioned melts.
Conclusions
The observed melts are characteristic of crystalline substances and suggest that the sorbitol crystallizes over time. During freezing, the excipient must remain in the same phase as the protein to ensure protein stability. By crystallizing, the sorbitol is phase-separated from the protein, which leads to protein aggregation.







Similar content being viewed by others
Abbreviations
- 10 mM sodium acetate, pH 4.0 with 300 mM sorbitol:
-
(A4S)
- 10 mM sodium acetate, pH 4.0 with 320 mM sucrose:
-
(A4Su)
- 10 mM sodium acetate, pH 5.0 with 300 mM sorbitol:
-
(A5S)
- differential scanning calorimetry:
-
(DSC)
- fragment crystallizes easily:
-
(Fc)
- glass transition temperature:
-
(Tg′)
- size exclusion high performance liquid chromatography:
-
(SE-HPLC)
References
T. W. Randolph. Phase separation of excipients during lyophilization: effects on protein stability. J. Pharm. Sci. 86:1198–1203 (1997).
P. L. Privalov. Cold denaturation of proteins. Crit. Rev. Biochem. Mol. Biol. 25:281–305 (1990).
F. Franks. Protein destabilization at low temperatures. Adv. Protein Chem. 46:105–139 (1995).
U. Hansonn. Aggregation of human immunoglobulin G upon freezing. Acta Chem. Scand. 22:483–489 (1968).
J. F. Carpenter, J. H. Crowe. The mechanism of cryoprotection of proteins by solutes. Cryobiology 25:244–255 (1988).
F. Franks. Solid aqueous solutions. Pure and Appl. Chem. 65:2527–2537 (1993).
L. L. Chang, D. Shepherd, J. Sun, D. Ouellette, K. L. Grant, X. C. Tang, and M. J. Pikal. Mechanism of protein stabilization by sugars during freeze-drying and storage: native structure preservation, specific interaction, and/or immobilization in a glassy matrix? J. Pharm. Sci. 94:1427–1444 (2005).
F. Franks. Freeze-drying: from empiricism to predictability. The significance of glass transitions. Dev. Biol. Stand. 74(discussion 19):9–18 (1992).
F. Franks, R. H. M. Hatley, and S. F. Mathias. Material science and the production of shelf-stable pharmaceuticals. BioPharm. 3:26–30 (1991).
M. Pikal. Freeze drying of proteins. In J. Cleland, Langer, R. (ed.), Stability, Formulation and Delivery of Peptides and Proteins, ACS Symposium Series, American Chemical Society, 1994, pp. 120–133.
J. F. Carpenter, S. J. Prestrelski, and T. Arakawa. Separation of freezing- and drying-induced denaturation of lyophilized proteins using stress-specific stabilization I. Enzyme activity and calorimetric studies. Arch. Biochem. Biophys. 303:456–464 (1993).
K. Izutsu, S. Yoshioka, and T. Terao. Decreased protein-stabilizing effects of cryoprotectants due to crystallization. Pharm. Res. 10:1232–1237 (1993).
F. Franks. Improved freeze drying: an analysis of the basic scientific principles. Process Biochem. 24:3–6 (1989).
L. van Den Berg and D. Rose. Effect of freezing on the pH and composition of sodium and potassium phosphate solutions; the reciprocal system KH2PO4–Na2–HPO4–H2O. Arch. Biochem. Biophys. 81:319–329 (1959).
G. B. Strambini and E. Gabellieri. Proteins in frozen solutions: evidence of ice-induced partial unfolding. Biophys. J. 70:971–976 (1996).
E. Gabellieri and G. B. Strambini. Perturbation of protein tertiary structure in frozen solutions revealed by 1-anilino-8-naphthalene sulfonate fluorescence. Biophys. J. 85:3214–3220 (2003).
L. Kreilgaard, L. S. Jones, T. W. Randolph, S. Frokjaer, J. M. Flink, M. C. Manning, and J. F. Carpenter. Effect of Tween 20 on freeze-thawing- and agitation-induced aggregation of recombinant human factor XIII. J. Pharm. Sci. 87:1597—1603 (1998).
B. S. Chang, B. S. Kendrick, and J. F. Carpenter. Surface-induced denaturation of proteins during freezing and its inhibition by surfactants. J. Pharm. Sci. 85:1325–1330 (1996).
J. C. Lee and S. N Timasheff. The stabilization of proteins by sucrose. J. Biol. Chem. 256:7193–7201 (1981).
R. Rowe, P. Sheskey, P. Weller, R. Rowe, P. Sheskey, and P. Weller. In R. C. Rowe. (ed.), Handbook of Pharmaceutical Excipients, 4th ed., American Pharmaceutical Association.
H. Levine and L. Slade. Thermomechanical properties of small-carbohydrate–water glasses and 'rubbers'. J. Chem. Soc., Faraday Trans. 84:2619–2633 (1988).
H. Costantino. Excipients for Use in Lyophilized Pharmaceutical Peptide, Protein and other Bioproducts. In H. Constantino, Pikal, M. (ed), Lyophilization of Biopharmaceuticals, AAPS, Arlington, 2004, pp. 139–228.
L. L. Chang, D. Shepherd, J. Sun, X. C. Tang, and M. J. Pikal. Effect of sorbitol and residual moisture on the stability of lyophilized antibodies: Implications for the mechanism of protein stabilization in the solid state. J. Pharm. Sci. 94:1445–1455 (2005).
L. M. Her, M. Deras, and S. L. Nail. Electrolyte-induced changes in glass transition temperatures of freeze-concentrated solutes. Pharm. Res. 12:768–772 (1995).
K. Izutsu, and S. Kojima. Freeze-concentration separates proteins and polymer excipients into different amorphous phases. Pharm. Res. 17:1316–1322 (2000).
S. Chongprasert, S. A. Knopp, and S. L. Nail. Characterization of frozen solutions of glycine. J. Pharm. Sci. 90:1720–1728 (2001).
K. Izutsu, S. Yoshioka, S. Kojima, T. W. Randolph, and J. F. Carpenter. Effects of sugars and polymers on crystallization of poly(ethylene glycol) in frozen solutions: phase separation between incompatible polymers. Pharm. Res. 13:1393–1400 (1996).
R. K. Cavatur, N. M. Vemuri, A. Pyne, Z. Chrzan, D. Toledo-Velasquez, and R. Suryanarayanan. Crystallization behavior of mannitol in frozen aqueous solutions. Pharm. Res. 19:894–900 (2002).
K. Izutsu, S. Yoshioka, and T. Terao. Effect of mannitol crystallinity on the stabilization of enzymes during freeze-drying. Chem. Pharm. Bull. 42:5–8 (1994).
J. K. Guillory. Generation of polymorphs, hydrates, solvates and amorphous solids. In H. G. Brittan (ed), Polymorphs in Pharmaceutical Solids. Marcel Decker, New York, 1999, pp. 183–226.
X. Liao, R. Krishnamurthy, and R. Suryanarayanan. Influence of the active pharmaceutical ingredient concentration on the physical state of mannitol-implications in freeze-drying. Pharm. Res. 22:1978–1985 (2005).
B. Chang, and C. Randall. Use of subambient thermal analysis to optimize protein lyophilization. Cryobiology 29:632–656 (1992).
L. Yu, S. Reutzel-Edens, and C. Mitchell. Crystallization and polymorphism of conformationally flexible molecules: problems, patterns and strategies. Org. Process Res. Dev. 4:396–402 (2000).
G. A. Jeffrey and H. S. Kim. Conformation of the Alditols. Carbohydr. Res. 14:207–216 (1970).
Acknowledgments
We thank Dr. Michael Treuheit, Dr. Vasumathi Dharmavaram, Alison Butler, Priti Parmar, Dr. Mary Elizabeth Wimer, Dr. David Brems and Dr. Susan Hershenson for a critical reading of the manuscript and support.
Deirdre Murphy Piedmonte and Christie Summers contributed equally to this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Piedmonte, D.M., Summers, C., McAuley, A. et al. Sorbitol Crystallization Can Lead to Protein Aggregation in Frozen Protein Formulations. Pharm Res 24, 136–146 (2007). https://doi.org/10.1007/s11095-006-9131-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-9131-1