Purpose
To demonstrate the utility of differential scanning calorimetry (DSC) for determining activation energy landscape in amorphous pharmaceutical systems throughout the sub-T g and T g regions.
Materials and Methods
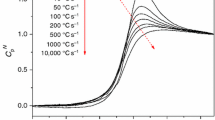
DSC was employed to determine the effective activation energies (E) of the relaxation in sub-T g and T g regions as well as the sizes of cooperatively rearranging regions in glassy maltitol and glucose.
Results
It has been found that in the sub-T g region E decreases with decreasing T reaching the values ∼60 (glucose) and ∼70 (maltitol) kJ mol−1 that are comparable to the literature values of the activation energies for the β-relaxation. In the T g region E decreases (from ∼250 to ∼150 kJ mol−1 in maltitol and from ∼220 to ∼170 kJ mol−1 in glucose) with increasing T as typically found for the α-relaxation. From the heat capacity measurements the sizes of cooperatively rearranging regions have been determined as 3.1 (maltitol) and 3.3 (glucose) nm.
Conclusions
DSC can be used for evaluating the energy landscapes. The E values for maltitol are somewhat greater than for glucose due to the added impeding effect of the bulky substitute group in maltitol. The comparable sizes of the cooperatively rearranging regions suggest a similarity of the heterogeneous glassy structures of the two compounds.









Similar content being viewed by others
References
E. Donth. The Glass Transition: Relaxation Dynamics in Liquids and Disordered Materials, Springer, Berlin, 2001.
C. A. Angell. Relaxation in liquids, polymers and plastic crystals—strong/fragile patterns and problems. J. Non-Cryst. Solids 131–133:13–31 (1991).
S. Vyazovkin, and I. Dranca. A DSC Study of α- and β-relaxations in a PS-clay system. J. Phys. Chem. B 108:11981–11987 (2004).
S. Vyazovkin, N. Sbirrazzuoli, and I. Dranca I. Variation of the effective activation energy throughout the glass transition. Macromol. Rapid Commun. 25:1708–1713 (2004).
B. Wunderlich. Thermal Analysis, Academic, Boston, 1990.
K.-H. Illers. Einfluss der termischen Vorgeschichte auf die Eigenschaften von Polyvinylchlorid. Makromol. Chem. 127:1–33 (1969).
H. S. Chen. On the mechanisms of structural relaxation in a Pd48Ni32P20 glass. J. Non-Cryst. Solids 46:289–305 (1981).
H. S. Chen. Kinetics of low temperature structural relaxation in two (Fe-Ni)-based metallic glasses. J. Appl. Phys. 52:1868–1870 (1981).
V. A. Bershtein and V. M. Egorov. Differential Scanning Calorimetry of Polymers, Ellis Horwood, New York, 1994.
V. A. Bershtein, and V. M. Yegorov. General mechanism of the β transition in polymers. Polym. Sci. USSR 27:2743–2757 (1985).
K. Kawai, T. Hagiwara, R. Takai, and T. Suzuki. Comparative investigation by two analytical approaches of enthalpy relaxation for glassy glucose, maltose, and trehalose. Pharm. Res. 22:490–495 (2005).
S. Vyazovkin, and I. Dranca. Probing beta relaxation in pharmaceutically relevant glasses by using DSC. Pharm. Res. 23:422–428 (2006).
L. Carpentier and M. Descamps. Dynamic decoupling and molecular complexity of glass-forming maltitol. J. Phys. Chem. B 107:271–275 (2003).
A. Faivre, G. Niquet, M. Maglione, J. Fornazero, J. F. Jal, and L. David. Dynamics of sorbitol and maltitol over a wide time-temperature range. Eur. Phys. J. B 10:277–286 (1999).
N. T. Correia, C. Alvarez, J. J. M. Ramos, and M. Descamps. Molecular motions in molecular glasses as studied by thermally stimulated depolarization currents (TSDC). Chem. Phys. 252:151–163 (2000).
T. R. Noel, R. Parker, and S. G. Ring. Effect of molecular structure and water content on the dielectric relaxation behaviour of amorphous low molecular weight carbohydrates above and below their glass transition. Carbohydr. Res. 329:839–845 (2000).
Gangasharan and S. S. N. Murthy. Nature of the relaxation processes in the suprecooled liquid and the glassy states of some carbohydrates. J. Phys. Chem. 99:12349–12354 (1995).
R. K. Chan, K. Pathmanathan, and G. P. Johari. Dielectric relaxations in the liquid and glassy states of glucose its water mixtures. J. Phys. Chem. 90:6358–6362 (1986).
A. Kudlik, S. Benkhof, T. Blochowicz, C. Tschirwitz, and E. Rössler. The dielectric response of simple organic glass formers. J. Mol. Str. 479:201–218 (1999).
C. T. Moynihan, A. J. Eastel, J. Wilder, and J. Tucker. Dependence of the glass transition temperature on heating and cooling rate. J. Phys. Chem. 78:2673–2677 (1974).
A. J. Kovacs, J. M. Hutchinson, and J. J. Aklonis, The Structure of Non-Crystalline Materials; P. H. Gaskell, Ed.; Taylor & Francis: 1977, p. 153.
S. Matsuoka. Relaxation Phenomena in Polymers, Hanser, Munich, 1992.
R. Bohmer, K. L. Ngai, C. A. Angell, and D. J. Plazek. Nonexponential relaxations in strong and fragile glass formers. J. Chem. Phys. 99:4201–4209 (1993).
T. Honma, Y. Benino, T. Komatsu, R. Sato, and V. Dimitrov. Structural relaxation kinetics of antimony borate glasses with covalent bonding character. J. Chem. Phys. 115:7207–7214 (2001).
C. A. Angell, R. C. Stell, and W. Sichina. Viscosity-temperature function for sorbitol from combined viscosity and differential scanning calorimetry studies. J. Phys. Chem. 86:1540–1542 (1982).
B. C. Hancock, C. R. Dalton, M. J. Pikal, and S. L. Shamblin. A pragmatic test of a simple calorimetric method for determining the fragility of some amorphous pharmaceutical materials. Pharm. Res. 15:762–767 (1998).
J. D. Ferry. Viscoelastic Properties of Polymers, 3rd ed. J. Wiley, New York, 1980.
S. Vyazovkin. Evaluation of the activation energy of thermally stimulated solid-state reactions under an arbitrary variation of the temperature. J. Comput. Chem. 18:393–402 (1997).
S. Vyazovkin. Modification of the integral isoconversional method to account for variation in the activation energy. J. Comput. Chem. 22:178–183 (2001).
I. M. Hodge. Enthalpy relaxation and recovery in amorphous materials J. Non-Cryst. Solids 169:211–266 (1994).
O. Bustin, and M. Descamps. Slow structural relaxations of glass-forming maltitol by modulated DSC calorimetry. J. Chem. Phys. 110:10982–10992 (1999).
R. Wungtanagorn, and S. J. Schmidt. Phenomenological study of enthalpy relaxation of amorphous glucose, fructose, and their mixture. Thermochim. Acta 369:95–116 (2001).
T. G. Fox, and P. J. Flory. Second-order transition temperatures and related properties of polystyrene. J. Appl. Phys. 21:581–591 (1950).
J. R. McLoughlin, and A. V. Tobolsky. The viscoelastic behavior of polymethyl methacrylate. J. Coll. Sci. 7:555–568 (1952).
A. Schouten, J. A. Kanters, J. Kroon, P. Looten, P. Duflot, and M. Mathlouthi. A redetermination of the crystal and molecular structure of maltitol (4-O-α-d-glucopyranosyl-d-glucitol). Carbohydr. Res. 322:298–302 (1999).
T. R. R. McDonald and C. A. Beevers. The crystal and molecular structure of α-glucose. Acta Cryst. 5:654–659 (1952).
E. Hempel, G. Hempel, A. Hensel, C. Schick, and E. Donth. Charcteristic length of dynamic glass transition near T g for a wide assortment of glass-forming substances. J. Phys. Chem. B 104:2460–2466 (2000).
P. D. Orford, R. Parker, and S. G. Ring. Aspects of the glass transition behaviour of mixtures of carbohydrates of low molecular weight. Carbohydr. Res. 196:11–18 (1990).
Acknowledgment
The authors are grateful to the Boehringer–Ingelheim Cares Foundation for support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vyazovkin, S., Dranca, I. Comparative Relaxation Dynamics of Glucose and Maltitol. Pharm Res 23, 2158–2164 (2006). https://doi.org/10.1007/s11095-006-9050-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-9050-1