Purpose
The purpose of this study was to evaluate the efficiency of a neuro-fuzzy logic-based methodology to model poorly soluble drug formulations and predict the development of the particle size that has been proven to be an important factor for long-term stability.
Methods
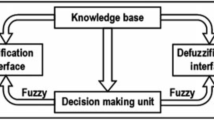
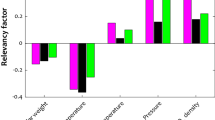
An adaptive neuro-fuzzy inference system was used to model the natural structures within the data and construct a set of fuzzy rules that can subsequently used as a predictive tool. The model was implemented in Matlab 6.5 and trained using 75% of an experimental data set. Subsequently, the model was evaluated and tested using the remaining 25%, and the predicted values of the particle size were compared to the ones from the experimental data. The produced adaptive neuro-fuzzy inference system-based model consisted of four inputs, i.e., acetone, propylene glycol, POE-5 phytosterol (BPS-5), and hydroxypropylmethylcellulose 90SH-50, with four membership functions each. Moreover, 256 fuzzy rules were employed in the model structure.
Results
Model training resulted in a root mean square error of 4.5 × 10−3, whereas model testing proved its highly predictive efficiency, achieving a correlation coefficient of 0.99 between the actual and the predicted values of the particle size (mean diameter).
Conclusions
Neuro-fuzzy modeling has been proven to be a realistic and promising tool for predicting the particle size of drug formulations with an easy and fast way, after proper training and testing.





Similar content being viewed by others
Abbreviations
- ANFIS:
-
adaptive neuro-fuzzy inference system
- ANN:
-
artificial neural network
- BPS-5:
-
PEG-5 soy sterol
- EDM:
-
empirical-data-based model
- FD:
-
factorial design
- FL:
-
fuzzy logic
- HPMC:
-
hydroxypropylmethylcellulose
- \(In^{i}_{j} {\left( k \right)}\) :
-
j = 1,2,...,N i
- \(k = 1,...,M_{i} ,i = 1,2i\) :
-
denotes the experimental phase, i.e., FD (i = 1) or RSM (i = 2), M i is the number of available data per causal factor, N i is the number of causal factors per experimental phase i, \(In^{i}_{j} {\left( k \right)}\) denotes the value of the causal factor j for the ith experimental phase and the kth experiment
- IT:
-
infusion technique
- M 25% :
-
the 25% of the available samples per causal factor denoting the size of the testing vector
- M 75% :
-
the 75% of the available samples per causal factor denoting the size of the training vector
- OXC:
-
oxcarbazepine
- PIDS:
-
polarization intensity differential scattering
- PSi(k), k = 1, ..., M i :
-
the particle size estimated at the ith experimental phase and the kth experiment
- PSANFIS(k), k = 1, ..., M25%:
-
the particle size estimated by ANFIS for the kth experiment that belongs to the testing vector
- PSANN(k), k = 1, ..., M25%:
-
the particle size estimated by ANN for the kth experiment that belongs to the testing vector
- RMSE:
-
root mean-square error
- RSM:
-
response surface methodology
References
J.-Y. Cherng H. Talsma R. Verrijk D. J. A. Crommelin W. E. Hennink (1999) ArticleTitleThe effect of formulation parameters on the size of poly-((2-dimethylamino)ethyl methacrylate)–plasmid complexes Eur. J. Pharm. Biopharm. 47 215–224 Occurrence Handle10382105 Occurrence Handle1:CAS:528:DyaK1MXktVajur0%3D Occurrence Handle10.1016/S0939-6411(98)00103-9
G. G. Agyralides P. P. Dallas D. M. Rekkas (2004) ArticleTitleDevelopment and in vitro evaluation of furosemide transdermal formulations using experimental design techniques Int. J. Pharm. 281 35–43 Occurrence Handle15288341 Occurrence Handle1:CAS:528:DC%2BD2cXmtFCmurY%3D Occurrence Handle10.1016/j.ijpharm.2004.05.011
Y. Miyamoto S. Ogawa M. Miyajima M. Matsui H. Sato K. Takayama T. Nagai (1997) ArticleTitleAn application of the computer optimization technique to wet granulation process involving explosive growth of particles Int. J. Pharm. 149 25–36 Occurrence Handle1:CAS:528:DyaK2sXjsVClu7c%3D Occurrence Handle10.1016/S0378-5173(96)04853-3
J. S. Chu G. L. Amidon N. D. Weiner A. H. Goldberg (1991) ArticleTitleMixture experimental design in the development of the muchoadhesive gel formulation Pharm. Res. 8 IssueID11 1401–1407 Occurrence Handle1798677 Occurrence Handle1:CAS:528:DyaK3MXms1egtL8%3D Occurrence Handle10.1023/A:1015853223929
J. M. Pean M. C. Venier-Julienne R. Filmon M. Sergent R. Phan-Tan-Luu J. P. Benoit (1998) ArticleTitleOptimization of HSA and NGF encapsulation yields in PLGA microparticles Int. J. Pharm. 166 105–115 Occurrence Handle1:CAS:528:DyaK1cXis1Wqsbw%3D Occurrence Handle10.1016/S0378-5173(98)00033-7
Y. M. Wang H. Sato I. Adachi I. Horicoshi (1996) ArticleTitleOptimization of the formulation design of chitosan microspheres containing Cisplatin J. Pharm. Sci. 85 IssueID11 1204–1210 Occurrence Handle8923326 Occurrence Handle1:CAS:528:DyaK28XmtFKhtr0%3D Occurrence Handle10.1021/js960092j
C. M. Sancho R. H. Vanrell S. Negro (2004) ArticleTitleOptimisation of aciclovir poly(d,l-lactide-co-glycolide) microspheres for intravitreal administration using a factorial design study Int. J. Pharm. 273 45–56 Occurrence Handle10.1016/j.ijpharm.2003.12.006
G. Derringer R. Suich (1980) ArticleTitleSimultaneous optimization of several response variables J. Qual. Technol. 12 214–219
A. I. Khuri M. Conlon (1981) ArticleTitleSimultaneous optimization of multiple responses by polynomial regression function Technometrics 23 365–375 Occurrence Handle10.2307/1268226
A. D. McLeod F. C. Lam P. K. Gupta C. T. Hung (1988) ArticleTitleOptimized synthesis of polyglutaraldehyde nanoparticles using central composite design J. Pharm. Sci. 77 704–710 Occurrence Handle3145338 Occurrence Handle1:CAS:528:DyaL1cXlsFKit7o%3D
B. G. Muller H. Leunberger T. Kissel (1996) ArticleTitleAlbumin nanospheres as carriers for passive drug targeting: an optimized manufacturing technique Pharm. Res. 13 IssueID1 32–37 Occurrence Handle8668675 Occurrence Handle1:STN:280:BymB387mslE%3D Occurrence Handle10.1023/A:1016064930502
D. C. Montgomery (2001) Response surface methods and other approaches to process optimization R. H. Myers D. C. Montgomery (Eds) Response Surface Methodology Wiley & Sons, Inc. New York 427–500
Y. Sun Y. Peng Y. Chen A. J. Shukla (2003) ArticleTitleApplication of artificial neural networks in the design of controlled release drug delivery systems Adv. Drug Deliv. Rev. 55 1201–1215 Occurrence Handle12954199 Occurrence Handle1:CAS:528:DC%2BD3sXmvVKqur0%3D Occurrence Handle10.1016/S0169-409X(03)00119-4
K. Takayama M. Fujikawa T. Nagai (1999) ArticleTitleArtificial neural network as a novel method to optimize pharmaceutical formulations Pharm. Res. 16 IssueID1 1–6 Occurrence Handle9950271 Occurrence Handle1:CAS:528:DyaK1MXmtlGntw%3D%3D
K. Takayama M. Fujikawa Y. Obata M. Morishita (2003) ArticleTitleNeural network based optimization of drug formulations Adv. Drug Deliv. Rev. 55 1217–1231 Occurrence Handle12954200 Occurrence Handle1:CAS:528:DC%2BD3sXmvVKquro%3D Occurrence Handle10.1016/S0169-409X(03)00120-0
A. S. Achanta J. G. Kowalski C. T. Rhodes (1995) ArticleTitleArtificial neural networks: implications for pharmaceutical sciences Drug Dev. Ind. Pharm. 21 119–155 Occurrence Handle1:CAS:528:DyaK2MXjtlGmsro%3D
J.-S. R. Jang (1993) ArticleTitleANFIS: adaptive-network-based fuzzy inference systems IEEE Trans. Syst. Man Cybern. 23 665–685 Occurrence Handle10.1109/21.256541
L. A. Zadeh (1965) ArticleTitleFuzzy sets Inf. Control 8 338–353 Occurrence Handle10.1016/S0019-9958(65)90241-X
A. K. Pannier R. M. Brand D. D. Jones (2003) ArticleTitleFuzzy modelling of skin permeability coefficients Pharm. Res. 20 IssueID2 143–148 Occurrence Handle12636150 Occurrence Handle1:CAS:528:DC%2BD3sXpslWjsA%3D%3D Occurrence Handle10.1023/A:1022273115847
T. J. Ross (1995) Fuzzy Logic with Engineering Applications McGraw-Hill, Inc. New York
T. Bernd M. Kleutges A. Kroll (1999) ArticleTitleNonlinear black box modelling—fuzzy networks versus neural networks Neural Comput. Appl. 8 151–162 Occurrence Handle10.1007/s005210050017
A. Kroll (1996) ArticleTitleIdentification of functional fuzzy models using multidimensional reference fuzzy sets Fuzzy Sets Syst. 80 149–158 Occurrence Handle10.1016/0165-0114(95)00140-9
G. L. Amidon H. Lunnernas V. P. Shah J. R. Crison (1995) ArticleTitleA theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vitro bioavailability Pharm. Res. 12 413–420 Occurrence Handle7617530 Occurrence Handle1:CAS:528:DyaK2MXksVGqur8%3D Occurrence Handle10.1023/A:1016212804288
M. Martinez L. Augsburger T. Johnston W. W. Jones (2002) ArticleTitleApplying the biopharmaceutics classification system to veterinary pharmaceutical products. Part I. Biopharmaceutics and formulation considerations Adv. Drug Deliv. Rev. 54 805–824 Occurrence Handle12363432 Occurrence Handle1:CAS:528:DC%2BD38XnsVSjtrg%3D Occurrence Handle10.1016/S0169-409X(02)00070-4
R. Löbenberg G. L. Amidon (2000) ArticleTitleModern bioavailability, bioequivalence and biopharmaceutics classification system. New scientific approaches to international regulatory standards Eur. J. Pharm. Biopharm. 50 3–12 Occurrence Handle10840189 Occurrence Handle10.1016/S0939-6411(00)00091-6
M. R. Violanto. Method for making uniformly particles from water-insoluble organic compounds. U.S. Pat. No. A-4,826,689.
A. S. Noyes W. R. Whitney (1897) ArticleTitleThe rate of solution of solid substances in their own solutions J. Am. Chem. Soc. 19 930–934 Occurrence Handle10.1021/ja02086a003
N. Rasenack B. W. Müller (2002) ArticleTitleDissolution rate enhancement by in-situ-micronization of poorly water-soluble drugs Pharm. Res. 19 IssueID12 1894–1900 Occurrence Handle12523671 Occurrence Handle1:CAS:528:DC%2BD38XpsVSjsbY%3D Occurrence Handle10.1023/A:1021410028371
N. Rasenack H. Steckel B. W. Muller (2003) ArticleTitleMicronization of anti-inflammatory drugs for pulmonary delivery by a controlled crystallization process J. Pharm. Sci. 92 IssueID1 35–44 Occurrence Handle12486680 Occurrence Handle1:CAS:528:DC%2BD3sXisVantg%3D%3D Occurrence Handle10.1002/jps.10274
S. L. Raghavan K. Schuessel A. Davis J. Hadgraft (2003) ArticleTitleFormation and stabilization of triclosan colloidal suspensions using supersaturated systems Int. J. Pharm. 261 153–158 Occurrence Handle12878404 Occurrence Handle1:CAS:528:DC%2BD3sXls1SqsL8%3D Occurrence Handle10.1016/S0378-5173(03)00299-0
G. Mie (1908) ArticleTitleBeiträge zur optik trüber medien, speziell kolloidaler metallösungen Ann. Phys. 25 377–445 Occurrence Handle1:CAS:528:DyaD1cXhtFWqtQ%3D%3D
U. M. Fayyad G. Piatetsky-Sapiro P. Smyth (1996) From data mining to knowledge discovery: an overview U. M. Fayyad G. Piatetsky-Sapiro P. Smyth R. Uthurusamy (Eds) Advances in Knowledge Discovery and Data Mining AAAI Press/MIT Press Menlo Park, CA 37–54
J.-S. R. Jang. Neuro-Fuzzy Modeling: architecture, analyses and applications, Ph.D. thesis, University of California, Berkeley, CA, 1992.
L. H. Tsoukalas (1997) Fuzzy and Neural Approaches in Engineering Wiley & Sons. Inc. New York
Fuzzy Logic Toolbox User's Guide. Version 3, The Mathworks, Inc., Natick Massachusetts, 2003.
Signal Processing Toolbox User's Guide. Version 3, The Mathworks, Inc., Natick Massachusetts, 2003.
M. T. Hagan M. Menhaj (1994) ArticleTitleTraining feedforward networks with the Marquardt algorithm IEEE Trans. Neural Netw. 5 IssueID6 989–993 Occurrence Handle10.1109/72.329697
Neural Networks Toolbox User's Guide. Version 3, The Mathworks, Inc., Natick Massachusetts, 2003.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Douroumis, D., Hadjileontiadis, L.J. & Fahr, A. Adaptive Neuro-Fuzzy Modeling of Poorly Soluble Drug Formulations. Pharm Res 23, 1157–1164 (2006). https://doi.org/10.1007/s11095-006-0021-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-0021-3