Abstract
Purpose.
The objectives of this work were 1) to develop a theoretical pharmacodynamic model that captures dynamic changes resulting from drug/therapy mediated P-glycoprotein (P-gp) induction and 2) to compare the pharmacodynamic outcomes of several doxorubicin (DOX) dosing schemes through simulations.
Methods.
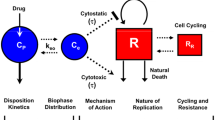
We developed a theoretical model that included a pharmacokinetic (PK) model for intracellular DOX-mediated P-gp induction and a pharmacodynamic (PD) model using a threshold trigger function for tumor cell-kill. In this model, both the level of P-gp induction and rate of tumor cell death were modulated by intracellular DOX concentration. Most model parameters were obtained from literature sources, and a few were either fixed or reasonably estimated.
Results.
Comparative dosing simulations showed that a 10-week constant infusion in which a tumor cell population was continuously exposed to the drug did not produce the best PD profile. On the other hand, dosing schemes where the cell population was initially challenged with a high dose, followed by intermittent dosing, generated the best PD profile. The favorable outcome of the latter dosing schemes was correlated with the lowest expression of P-gp in terms of area under the curve (AUC) during treatment period.
Conclusions.
The simulations led us to conclude that drug resistance, particularly resistance caused by P-gp overexpression, induced during chemotherapy may, in part, be circumvented by designing optimal dosing strategies that minimize P-gp induction.
Similar content being viewed by others
References
1. C. Marbeuf-Gueye, M. Salerno, P. Quidu, and A. Garnier-Suillerot. Inhibition of the P-glycoprotein- and multidrug resistance protein-mediated efflux of anthracyclines and calceinacetoxymethyl ester by PAK-104P. Eur. J. Pharmacol. 391:207–216 (2000).
2. J. Meesungnoen, J. P. Jay-Gerin, and S. Mankhetkorn. Relation between MDR1 mRNA levels, resistance factor, and the efficiency of P-glycoprotein-mediated efflux of pirarubicin in multidrug-resistant K562 sublines. Can. J. Physiol. Pharmacol. 80:1054–1063 (2002).
3. R. Maitra, P. A. Halpin, K. H. Karlson, R. L. Page, D. Y. Paik, M. O. Leavitt, B. D. Moyer, B. A. Stanton, and J. W. Hamilton. Differential effects of mitomycin C and doxorubicin on P-glycoprotein expression. Biochem. J. 355:617–624 (2001).
4. M. Demeule, M. Brossard, and R. Beliveau. Cisplatin induces renal expression of P-glycoprotein and canalicular multispecific organic anion transporter. Am. J. Physiol. 277:F832–F840 (1999).
5. X. Bian, T. D. Giordano, J. J. Lin, G. Solomon, V. P. Castle, and A. W. Opipari. Chemotherapy-induced apoptosis of S-type neuroblastoma cells requires caspase-9 and is augmented by CD95/Fas stimulation. J. Biol. Chem. 279:4663–4669 (2004).
6. L. Lothstein, M. Israel, and T. W. Sweatman. Anthracycline drug targeting: cytoplasmic versus nuclear—a fork in the road. Drug Resist. Update 4:169–177 (2001).
7. S. Fulda, H. Sieverts, C. Friesen, I. Herr, and K. M. Debatin. The CD95 (APO-1/Fas) system mediates drug-induced apoptosis in neuroblastoma cells. Cancer Res. 57:3823–3829 (1997).
8. M. S. Dordal, A. C. Ho, S. M. Jackson, Y. F. Fu, C. L. Goolsby, and J. N. Winter. Flow cytometric assessment of the cellular pharmacokinetics of fluorescent drugs. Cytometry 20:307–314 (1995).
9. T. L. Jackson. Intracellular accumulation and mechanism of action of doxorubicin in a spatio-temporal tumor model. J. Theor. Biol. 220:201–213 (2003).
10. W. L. Jusko. A pharmacodynamic Model for cell-cycle-cpecific chemotherapeutic agents. J. Pharmacokinet. Biopharm. 1:175–199 (1973).
11. P. R. Wielinga, H. V. Westerhoff, and J. Lankelma. The relative importance of passive and P-glycoprotein mediated anthracycline efflux from multidrug-resistant cells. Eur. J. Biochem. 267:649–657 (2000).
12. H. Harashima, S. Iida, Y. Urakami, M. Tsuchihashi, and H. Kiwada. Optimization of antitumor effect of liposomally encapsulated doxorubicin based on simulations by pharmacokinetic/pharmacodynamic modeling. J. Control. Rel. 61:93–106 (1999).
13. E. S. Lipshultz. Female sex and higher drug dose as risk factors for cardiotoxic effects of doxorubicin therapy for childhood cancer. N. Engl. J. Med. 26:332 (1995).
14. B. Orthan. Doxorubicin cardiotoxicity: growing importance. J. Clin. Oncol. 17:2294–2296 (1999).
15. P. Spallarossa, S. Garibaldi, P. Altieri, V. Manca, S. Nasti, P. Rossettin, G. Ghigliotti, A. Ballestrero, F. Patrone, A. Barsotti, and C. Brunelli. Carvedilol prevents doxorubicin-induced free radical release and apoptosis in cardiomyocytes in vitro. J. Mol. Cell. Cardiol. 37:837–846 (2004).
16. E. A. Perez. Paclitaxel and cardiotoxicity. J. Clin. Oncol. 16:3481–3482 (1998).
17. E. Crivellato, L. Candussio, A. M. Rosati, G. Decorti, F. B. Klugmann, and F. Mallardi. Kinetics of doxorubicin handling in the LLC-PK1 kidney epithelial cell line is mediated by both vesicle formation and P-glycoprotein drug transport. Histochem. J. 31:635–643 (1999).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luu, K., Uchizono, J. P-Glycoprotein Induction and Tumor Cell-Kill Dynamics in Response to Differential Doxorubicin Dosing Strategies: A Theoretical Pharmacodynamic Model. Pharm Res 22, 710–715 (2005). https://doi.org/10.1007/s11095-005-2585-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-005-2585-8