No Heading
Purpose.
Loperamide-induced suppressive effects on central nervous system closely relate to a lack of or decline in the P-glycoprotein (P-gp) function. The aim of this study was to determine the loperamide-induced sedative effect quantitatively and to investigate possible alterations in the pharmacokinetics of digoxin, a substrate for P-gp, in Japanese subjects.
Methods.
Loperamide hydrochloride (2 mg) was administered orally to 26 subjects and the critical flicker-fusion frequency threshold (CFF) values were measured every 30 min separately by portable instrument. Further, digoxin (0.25 mg) was administered to 8 subjects, and the plasma concentration was determined.
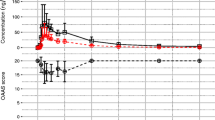
Results.
In five subjects who complained of drowsiness, the CFF values more remarkably decreased compared with those in the other subjects. The Tmax and mean residence time (MRT) values of digoxin pharmacokinetics in four subjects with drowsiness were significantly lower and Cmax was higher than those in four subjects with marginal effect. Moreover, there were good correlations between the CFF value-time profile and the Cmax, Tmax, and MRT of digoxin.
Conclusions.
The determination of the CFF value after oral administration of loperamide will be useful for evaluating varied P-gp function and for anticipating individual variations in the disposition of P-gp substrates in humans.
Similar content being viewed by others
Abbreviations
- AUC:
-
area under the digoxin concentration curve
- Cmax:
-
maximum plasma concentration
- CFF:
-
critical flicker-fusion frequency threshold
- CLtot/F:
-
total clearance divided by bioavailability
- CNS:
-
central nervous system
- CYP:
-
cytochrome P-450
- ΔCFF-AUC:
-
area under the ΔCFF curve
- Ka:
-
absorption constant
- Ke:
-
elimination constant
- MRT:
-
mean residence time
- P-gp:
-
P-glycoprotein
- Tmax:
-
time at maximum plasma concentration
References
1. P. Oelkers, L. C. Kirby, J. E. Heubi, and P. A. Dawson. Primary bile acid malabsorption caused by mutations in the ileal sodium-dependent bile acid transporter gene (SLC10A2). J. Clin. Invest. 99:1880–1887 (1997).
2. E. M. Wright. Genetic disorders of membrane transport I. Glucose galactose malabsorption. Am. J. Physiol. 275:G879–G882 (1998).
3. H. Tsujii, J. Konig, D. Rost, B. Stockel, U. Leuschner, and D. Keppler. Exon-intron organization of the human multidrug-resistance protein 2 (MRP2) gene mutated in Dubin-Johnson syndrome. Gastroenterology 117:653–660 (1999).
4. L. R. Schiller, C. A. Santa Ana, S. G. Morawski, and J. S. Fordtran. Mechanism of the antidiarrheal effect of loperamide. Gastroenterology 86:1475–1480 (1984).
5. J. Heykants, M. Michiels, A. Knaeps, and J. Brugmans. Loperamide (R 18 553), a novel type of antidiarrheal agent. Part 5: the pharmacokinetics of loperamide in rats and man. Arzneimittelforschung 24:1649–1653 (1974).
6. A. H. Schinkel, E. Wagenaar, C. A. Mol, and L. van Deemter. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J. Clin. Invest. 97:2517–2524 (1996).
7. A. J. Sadeque, C. Wandel, H. He, S. Shah, and A. J. Wood. Increased drug delivery to the brain by P-glycoprotein inhibition. Clin. Pharmacol. Ther. 68:231–237 (2000).
8. I. Hindmarch and A. C. Parrott. A repeated dose comparison of the side effects of five antihistamines on objective assessments of psychomotor performance, central nervous system arousal and subjective appraisals of sleep and early morning behaviour. Arzneimittelforschung 28:483–486 (1978).
9. I. Hindmarch and Z. Subhan. The effects of midazolam in conjunction with alcohol on sleep, psychomotor performance and car driving ability. Int. J. Clin. Pharmacol. Res. 3:323–329 (1983).
10. Z. Subhan and I. Hindmarch. The effects of lormetazepam on aspects of sleep and early morning performance. Eur. J. Clin. Pharmacol. 25:47–51 (1983).
11. J. L. Pretorius, M. Phillips, R. W. Langley, E. Szabadi, and C. M. Bradshaw. Comparison of clozapine and haloperidol on some autonomic and psychomotor functions, and on serum prolactin concentration, in healthy subjects. Br. J. Clin. Pharmacol. 52:322–326 (2001).
12. J. A. Endicott and V. Ling. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu. Rev. Biochem. 58:137–171 (1989).
13. S. F. Su and J. D. Huang. Inhibition of the intestinal digoxin absorption and exsorption by quinidine. Drug Metab. Dispos. 24:142–147 (1996).
14. Y. Tanigawara, N. Okamura, M. Hirai, M. Yasuhara, K. Ueda, N. Kioka, T. Komano, and R. Hori. Transport of digoxin by human P-glycoprotein expressed in a porcine kidney epithelial cell line (LLC-PK1). J. Pharmacol. Exp. Ther. 263:840–845 (1992).
15. C. Marzolini, E. Paus, T. Buclin, and R. B. Kim. Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevance. Clin. Pharmacol. Ther. 75:13–33 (2004).
16. S. Hoffmeyer, O. Burk, O. von Richter, H. P. Arnold, J. Brockmoller, A. Johne, I. Cascorbi, T. Gerloff, I. Roots, M. Eichelbaum, and U. Brinkmann. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc. Natl. Acad. Sci. USA 97:3473–3478 (2000).
17. A. Johne, K. Kopke, T. Gerloff, I. Mai, S. Rietbrock, C. Meisel, S. Hoffmeyer, R. Kerb, M. F. Fromm, U. Brinkmann, M. Eichelbaum, J. Brockmoller, I. Cascorbi, and I. Roots. Modulation of steady-state kinetics of digoxin by haplotypes of the P-glycoprotein MDR1 gene. Clin. Pharmacol. Ther. 72:584–594 (2002).
18. Y. Kurata, I. Ieiri, M. Kimura, T. Morita, S. Irie, A. Urae, S. Ohdo, H. Ohtani, Y. Sawada, S. Higuchi, and K. Otsubo. Role of human MDR1 gene polymorphism in bioavailability and interaction of digoxin, a substrate of P-glycoprotein. Clin. Pharmacol. Ther. 72:209–219 (2002).
19. C. Verstuyft, S. Strabach, H. El-Morabet, R. Kerb, U. Brinkmann, L. Dubert, P. Jaillon, C. Funck-Brentano, G. Trugnan, and L. Becquemont. Dipyridamole enhances digoxin bioavailability via P-glycoprotein inhibition. Clin. Pharmacol. Ther. 73:51–60 (2003).
20. T. Sakaeda, T. Nakamura, M. Horinouchi, M. Kakumoto, N. Ohmoto, T. Sakai, Y. Morita, T. Tamura, N. Aoyama, M. Hirai, M. Kasuga, and K. Okumura. MDR1 genotype-related pharmacokinetics of digoxin after single oral administration in healthy Japanese subjects. Pharm. Res. 18:1400–1404 (2001).
21. T. Nakamura, T. Sakaeda, M. Horinouchi, T. Tamura, N. Aoyama, T. Shirakawa, M. Matsuo, M. Kasuga, and K. Okumura. Effect of the mutation (C3435T) at exon 26 of the MDR1 gene on expression level of MDR1 messenger ribonucleic acid in duodenal enterocytes of healthy Japanese subjects. Clin. Pharmacol. Ther. 71:297–303 (2002).
22. T. Gerloff, M. Schaefer, A. Johne, K. Oselin, C. Meisel, I. Cascorbi, and I. Roots. MDR1 genotypes do not influence the absorption of a single oral dose of 1 mg digoxin in healthy white males. Br. J. Clin. Pharmacol. 54:610–616 (2002).
23. K. Yamaoka, Y. Tanigawara, T. Nakagawa, and T. Uno. A pharmacokinetic analysis program (multi) for microcomputer. J. Pharmacobiodyn. 4:879–885 (1981).
24. K. Tabata, K. Yamaoka, A. Kaibara, S. Suzuki, M. Terakawa, and T. Hata. Moment analysis program available on Microsoft Excel®. Xenobio Metabol. Dispos. 14:286–293 (1999).
25. K. Lauritsen, L. S. Laursen, and J. Rask-Madsen. Clinical pharmacokinetics of drugs used in the treatment of gastrointestinal diseases (Part II). Clin. Pharmacokinet. 19:94–125 (1990).
26. Y. Tayrouz, B. Ganssmann, R. Ding, A. Klingmann, R. Aderjan, J. Burhenne, W. E. Haefeli, and G. Mikus. Ritonavir increases loperamide plasma concentrations without evidence for P-glycoprotein involvement. Clin. Pharmacol. Ther. 70:405–414 (2001).
27. C. Wandel, R. Kim, M. Wood, and A. Wood. Interaction of morphine, fentanyl, sufentanil, alfentanil, and loperamide with the efflux drug transporter P-glycoprotein. Anesthesiology 96:913–920 (2002).
28. L. Becquemont, C. Verstuyft, R. Kerb, U. Brinkmann, M. Lebot, P. Jaillon, and C. Funck-Brentano. Effect of grapefruit juice on digoxin pharmacokinetics in humans. Clin. Pharmacol. Ther. 70:311–316 (2001).
29. P. Pauli-Magnus, J. Feiner, C. Brett, E. Lin, and D. L. Kroetz. No effect of MDR1 C3435T variant on loperamide disposition and central nervous system effects. Clin. Pharmacol. Ther. 74:487–498 (2003).
30. N. Morita, T. Yasumori, and K. Nakayama. Human MDR1 polymorphism: G2677T/A and C3435T have not effect on MDR1 transport activity. Biochem. J. 65:1843–1852 (2003).
31. B. Greiner, M. Eichelbaum, P. Fritz, H. P. Kreichgauer, O. von Richter, J. Zundler, and H. K. Kroemer. The role of intestinal P-glycoprotein in the interaction of digoxin and rifampin. J. Clin. Invest. 104:147–153 (1999).
32. D. Dürr, B. Stieger, G. A. Kullak-Ublick, K. M. Rentsch, H. C. Steinert, P. J. Meier, and K. Fattinger. St John’s Wort induces intestinal P-glycoprotein/MDR1 and intestinal and hepatic CYP3A4. Clin. Pharmacol. Ther. 68:598–604 (2000).
33. A. Yamauchi, I. Ieiri, Y. Kataoka, M. Tanabe, T. Nishizaki, R. Oishi, S. Higuchi, K. Otsubo, and K. Sugimachi. Neurotoxicity induced by tacrolimus after liver transplantation: relation to genetic polymorphisms of the ABCB1 (MDR1) gene. Transplantation 74:571–572 (2002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kobayashi, M., Saitoh, H., Yamaguchi, M. et al. Relationship Between Loperamide-Induced Sedative Effect and Digoxin Pharmacokinetics in Healthy Japanese Subjects. Pharm Res 22, 413–418 (2005). https://doi.org/10.1007/s11095-004-1879-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-004-1879-6