No Heading
Purpose.
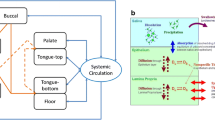
This study was aimed to develop a family of compartmental models to describe in a strictly quantitative manner the transdermal iontophoretic transport of drugs in vivo. The new models are based on previously proposed compartmental models for the transport in vitro.
Methods.
The novel in vivo model considers two separate models to describe the input into the systemic circulation: a) constant input and b) time-variant input. Analogous to the in vitro models, the in vivo models contain four parameters: 1) kinetic lag time (t L ), 2) steady-state flux during iontophoresis (J ss ), 3) skin release rate constant (K R ), and 4) passive flux in the post-iontophoretic period (J pas ). The elimination from the systemic circulation is described by a) the one-compartment and b) the two-compartment pharmacokinetic models. The models were applied to characterize the observed plasma concentration vs. time data following single-dose iontophoretic delivery of growth hormone-releasing factor (GRF) and R-apomorphine. Moreover, the models were also used to simulate the observed plasma concentration vs. time profiles following a two-dose transdermal iontophoretic administration of alniditan.
Results.
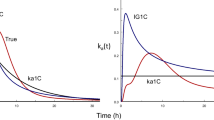
The time-variant input models were superior to the constant input models and appropriately converged to the observed data of GRF and R-apomorphine allowing the estimation of J ss , K R , and J pas . In most cases, the values of t L were negligible. The estimated J ss and the in vivo flux profiles of GRF and R-apomorphine were similar to those obtained using the deconvolution method. The two-dose iontophoretic transport of alniditan was properly simulated using the proposed time-variant input model indicating the utility of the model to predict and to simulate the drug transport by a multiple-dose iontophoresis. Moreover, the use of the compartmental modeling approach to derive an in vitro-in vivo correlation for R-apomorphine was demonstrated. This approach was also used to identify the optimum in vitro model that closely mimics the in vivo iontophoretic transport of R-apomorphine.
Conclusions.
The developed in vivo models demonstrate their consistency and capability to describe the in vivo iontophoretic drug transport. This compartmental modeling approach provides a scientific basis to examine in vitro-in vivo correlations of drug transport by iontophoresis.
Similar content being viewed by others
Abbreviations
- α and β:
-
micro constants obtained during integration/Laplace transformation
- AT:
-
the amount of drug presence in the central compartment at current removal
- A2T:
-
the amount of drug presence in the peripheral compartment at current removal
- A(t):
-
drug amount in plasma at time t
- Cp(t):
-
drug concentration in the central compartment (plasma) at time t
- DHS:
-
dermatomed human skin
- EF:
-
enhancement factor
- HSC:
-
human stratum corneum
- I0:
-
the constant rate of iontophoretic drug input into the skin
- IT:
-
the maximum iontophoretic drug input at current removal at time T
- Jss:
-
steady-state flux
- J(t):
-
flux at time t
- k:
-
the rate constant of drug elimination from the central compartment
- k12:
-
the rate constant of drug distribution from the central to the peripheral compartment
- k21:
-
the rate constant of drug distribution from the peripheral to the central compartment
- KR:
-
the rate constant of drug release from the skin into the systemic circulation (in vivo) or into the acceptor phase (in vitro)
- PPI:
-
the zero order mass input into the skin due to passive diffusion post-iontophoresis
- S:
-
patch area
- T:
-
time of current removal
- t’:
-
the net time post iontophoresis
- tL:
-
the kinetic lag time of the drug molecules to enter the skin compartment
- tN:
-
the net time of current application
- VD1:
-
volume of distribution of the central compartment
References
1. A. K. Nugroho, O. Della-Pasqua, M. Danhof, and J. A. Bouwstra. Compartmental modeling of transdermal iontophoretic transport: I. In vitro model derivation and application. Pharm. Res. 21:1974–1984 (2004).
2. G. L. Li, M. Danhof, P. M. Frederik, and J. A. Bouwstra. Pre-treatment with a water-based surfactant formulation affects transdermal iontophoretic delivery of R-apomorphine in vitro. Pharm. Res. 20:653–659 (2003).
3. M. S. Roberts, P. M. Lai, and Y. G. Anissimov. Epidermal iontophoresis: I. Development of the ionic mobility-pore model. Pharm. Res. 15:1569–1578 (1998).
4. P. M. Lai and M. S. Roberts. Epidermal iontophoresis: II. Application of the ionic mobility-pore model to the transport of local anesthetics. Pharm. Res. 15:1579–1588 (1998).
5. J. B. Phipps and J. R. Gyory. Transdermal ion migration. Adv. Drug Deliv. Rev. 9:137–176 (1992).
6. M. J. Pikal. Transport mechanisms in iontophoresis. I. A theoretical model for the effect of electroosmotic flow on flux enhancement in transdermal iontophoresis. Pharm. Res. 7:118–126 (1990).
7. M. J. Pikal and S. Shah. Transport mechanisms in iontophoresis. II. Electroosmotic flow and transference number measurements for hairless mouse skin. Pharm. Res. 7:213–221 (1990).
8. M. J. Pikal and S. Shah. Transport mechanisms in iontophoresis. III. An experimental study of the contributions of electroosmotic flow and permeability change in transport of low and high molecular weight solutes. Pharm. Res. 7:222–229 (1990).
9. J. E. Riviere, B. Sage, and P. L. Williams. Effects of vasoactive drugs on transdermal lidocaine iontophoresis. J. Pharm. Sci. 80:615–620 (1991).
10. J. E. Riviere, P. L. Williams, R. S. Hillman, and L. M. Mishky Quantitative prediction of transdermal iontophoretic delivery of arbutamine in humans with the in vitro isolated perfused porcine skin flap. J. Pharm. Sci. 81:504–507 (1992).
11. M. C. Heit, P. L. Williams, F. L. Jayes, S. K. Chang, and J. E. Riviere. Transdermal iontophoretic peptide delivery: in vitro and in vivo studies with luteinizing hormone releasing hormone. J. Pharm. Sci. 82:240–243 (1993).
12. M. Gibaldi and D. Perrier. Pharmacokinetics. Marcel Dekker, New York, 1982.
13. P. Singh, M. S. Roberts, and H. I. Maibach. Modelling of plasma levels of drugs following transdermal iontophoresis. J. Control. Rel. 33:293–298 (1995).
14. S. Kumar, H. Char, S. Patel, D. Piemontese, A. W. Malick, K. Iqbal, E. Neugroschel, and C. R. Behl. In vivo transdermal iontophoretic delivery of growth hormone releasing factor GRF (1–44) in hairless guinea pigs. J. Control. Rel. 18:213–220 (1992).
15. G. L. Li. Transdermal Iontophoretic Delivery of R-Apomorphine for the Treatment of Patients with Parkinson’s Disease. Ph.D. Thesis, Leiden University, Leiden, The Netherlands, 2003.
16. A. Jadoul, J. Mesens, W. Caers, F. de Beukelaar, R. Crabbe, and V. Preat. Transdermal permeation of alniditan by iontophoresis: in vitro optimization and human pharmacokinetic data. Pharm. Res. 13:1348–1353 (1996).
17. WinNonlin Professional v. 4.1. Pharsight Corporation, Mountain View, CA. (2003).
18. G. L. Li, A. Grossklaus, M. Danhof, and J. A. Bouwstra. Iontophoretic R-apomorphine delivery in combination with surfactant pretreatment: in vitro validation studies. Int. J. Pharm. 266:61–68 (2003).
19. R. Van der Geest, T. Van Laar, J. M. Gubbens-Stibbe, H. E. Bodde, and M. Danhof. Iontophoretic delivery of apomorphine. II: An in vivo study in patients with Parkinson’s disease. Pharm. Res. 14:1804–1810 (1997).
20. A. K. Nugroho, G. L. Li, M. Danhof, and J. A. Bouwstra. Transdermal iontophoresis of rotigotine across human stratum corneum in vitro: influence of pH and NaCl concentration. Pharm. Res. 21:844–850 (2004).
21. R. Van der Geest, M. Danhof, and H. E. Bodde. Iontophoretic delivery of apomorphine. I: In vitro optimization and validation. Pharm. Res. 14:1798–1803 (1997).
22. L. Finkelstein and E. R. Carson. Mathematical Modeling of Dynamic Biological Systems, 2nd ed. John Wiley & Sons, New York, 1985.
23. S. Grond, L. Radbruch, and K. A. Lehmann. Clinical pharmacokinetics of transdermal opioids: focus on transdermal fentanyl. Clin. Pharmacokinet. 38:59–89 (2000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nugroho, A., Della-Pasqua, O., Danhof, M. et al. Compartmental Modeling of Transdermal Iontophoretic Transport II: In Vivo Model Derivation and Application. Pharm Res 22, 335–346 (2005). https://doi.org/10.1007/s11095-004-1870-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-004-1870-2