No Heading
Purpose.
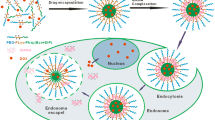
To evaluate a new polymeric nanoparticulate drug delivery formulation that consists of two components: i) an amphiphilic diblock copolymer having tocopherol moiety at the end of the hydrophobic block in which the hydrophobic tocopherol moiety increases stability of hydrophobic core of the nanoparticle in aqueous medium; and ii) a biodegradable copolyester having carboxylate end group that is capable of forming ionic complex with positively charged compounds such as doxorubicin.
Methods.
A doxourubicin-loaded polymeric nanoparticle (Dox-PNP) was prepared by solvent evaporation method. The entrapment efficiency, size distribution, and in vitro release profile at various pH conditions were characterized. In vitro cellular uptake was investigated by confocal microscopy, flow cytometry, and MTT assay using drug-sensitive and drug-resistant cell lines. Pharmacokinetics and biodistribution were evaluated in rats and tumor-bearing mice.
Results.
Doxorubicin (Dox) was efficiently loaded into the PNP (higher than 95% of entrapment efficiency), and the diameter of Dox-PNP was in the range 20~25 nm with a narrow size distribution. In Vitro study showed that Dox-PNP exhibited higher cellular uptake into both human breast cancer cell (MCF-7) and human uterine cancer cell (MES-SA) than free doxorubicin solution (Free-Dox), especially into drug-resistant cells (MCF-7/ADR and MES-SA/Dx-5). In pharmacokinetics and tissue distribution study, the bioavailability of Dox-PNP calculated from the area under the blood concentration-time curve (AUC) was 69.8 times higher than that of Free-Dox in rats, and Dox-PNP exhibited 2 times higher bioavailability in tumor tissue of tumor-bearing mice.
Conclusions.
Dox-PNP exhibited enhanced cellular uptake of the drug. In the cytotoxic activity study, this improved cellular uptake was proved to be more advantageous in drug-resistant cell. Dox-PNP exhibited much higher bioavailability in blood plasma and more drug accumulation in tumor tissue than conventional doxorubicin formulation. The results of this study suggest that the PNP system is an advantageous carrier for drug delivery.
Similar content being viewed by others
Abbreviations
- Dox-PNP:
-
doxorubicin-containing polymeric nanoparticle
- Free-Dox:
-
aqueous solution of doxorubicin hydrochloride
- mPEG-PLA-Toco:
-
methoxy poly(ethylene glycol)-b-poly(lactic acid) having a tocopherol moiety at the end of hydrophobic block
- PLMA-COONa:
-
sodium salt of poly (lactic acid-co-mandelic acid)
- PNP:
-
polymeric nanoparticle
References
1. V. P. Torchilin and V. S. Trubetskoy. Which polymers can make nanoparticulate drug carriers long-circulating? Adv. Drug Deliv. Rev. 16:141–155 (1995).
2. M.-C. Jones and J.-C. Leroux. Polymeric micelles—a new generation of colloidal drug carriers. Eur. J. Pharm. Biopharm. 48:101–111 (1999).
3. J. Panyam and V. Labhasetwar. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 55:329–347 (2003).
4. K. Kataoka, T. Matsumoto, M. Yokoyama, T. Okano, Y. Sakurai, S. Fukushima, K. Okamoto, and G. S. Kwon. Doxorubicin-loaded poly(ethylene glycol)-poly(β-benzyl-L-aspartate) copolymer micelles: their pharmaceutical characteristics and biological significance. J. Control. Rel. 64:143–153 (2000).
5. V. Alakhov, E. Klinski, S. Li, G. Pietrzynski, A. Venne, E. Batrakova, T. Bronitch, and A. Kabanov. Block-copolymer-based formulation of doxorubicin. From cell screen to clinical trials. Colloids Surf. B Biointerfaces 16:113–134 (1999).
6. G. M. Barratt. Therapeutic applications of colloidal drug carriers. Pharm. Sci. Technol. Today 3:163–171 (2000).
7. V. Torchilin. Structure and design of polymeric surfactant-based drug delivery systems. J. Control. Rel. 73:137–172 (2001).
8. H. Maeda. The tumor blood vessel as an ideal target for macromolecular anticancer agents. J. Control. Rel. 19:315–324 (1992).
9. T. N. Palmer, V. J. Caride, M. A. Caldecourt, J. Twickler, and V. Abdullah. The mechanism of liposome accumulation in infarction. Biochim. Biophys. Acta 797:363–368 (1984).
10. A. A. Gabizon. Liposome circulation time and tumor targeting: implications for cancer chemotherapy. Adv. Drug Deliv. Rev. 16:285–294 (1995).
11. H. Maeda, J. Wu, T. Sawa, Y. Matsumura, and K. Hori. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Control. Rel. 65:271–284 (2000).
12. K. J. Zhu, L. Xiangzhou, and Y. Shinlin. Preparation, characterization, and properties of polylactide (PLA)-poly(ethylene glycol) (PEG) copolymers: A potential drug carrier. J. Appl. Polym. Sci. 39:1–9 (1990).
13. B. Jeong, Y. H. Bae, D. S. Lee, and S. W. Kim. Biodegradable block copolymers as injectable drug-delivery systems. Nature 388:860–862 (1997).
14. P. R. Walker, J. Kwast-Welfeld, H. Gourdeau, J. Leblanc, W. Neugebaur, and M. Sikorska. Relationship between apoptosis and the cell cycle in lymphocytes: roles of protein kinase C, tyrosine phosphorylation, and AP1. Exp. Cell Res. 207:142–151 (1993).
15. Y. Kakizawa and K. Kataoka. Block copolymer micelles for delivery of gene and related compounds. Adv. Drug Deliv. Rev. 54:203–222 (2002).
16. I. Brigger, C. Dubernet, and P. Couvreur. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 54:631–651 (2002).
17. Y. Bae, S. Fukushima, A. Harada, and K. Kataoka. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: polymeric micelles that are responsive to intracellular pH change. Angew. Chem. Int. Ed. Engl. 42:4640–4643 (2003).
18. J. Panyam, W.-Z. Zhou, S. Prabha, S. K. Sahoo, and V. Labhasetwar. Rapid endo-lysosomal escape of poly(DL-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. FASEB J. 16:1217–1226 (2002).
19. V. Omelyanenko, P. Kopečková, C. Gentra, and J. Kopeček. Targetable HPMA copolymer-adriamycin conjugate. Recognition, internalization, and subcellular fate. J. Control. Rel. 53:25–37 (1998).
20. D. Nielsen, C. Maare, and T. Skovsgaard. Cellular resistance to anthracyclines. Gen. Pharmacol. 27:251–255 (1996).
21. L. M. Leoni, E. Hamel, D. Genini, H. Shih, C. J. Carrera, H. B. Cottam, and D. A. Carson. Indanocine, a microtubule-binding indanone and a selective inducer of apoptosis in multidrug-resistant cancer cells. J. Natl. Cancer Inst. 92:217–224 (2000).
22. D. A. Scudiero, A. Monks, and E. A. Sausville. Cell line designation change: Multidrug-resistant cell line in the NCI anticancer screen. J. Natl. Cancer Inst. 90:862 (1998).
23. H. Lage and M. Dietel. Effect of the breast-cancer resistance protein on atypical multidrug resistance. Lancet Oncol. 1:169–175 (2000).
24. D. D. Ross, W. Yang, L. V. Abruzzo, W. S. Dalton, E. Schneider, H. Lage, M. Dietel, L. Greenberger, S. P. C. Cole, and L. A. Doyle. Atypical multidrug resistance: breast cancer resistance protein messenger RNA expression in mitoxantrone-selected cell lines. J. Natl. Cancer Inst. 91:429–433 (1999).
25. A. J. Vander, J. H. Sherman, and D. S. Luciano. Movements of molecules across cell membranes. In A. J. Vander, J. H. Sherman, and D.S. Luciano (eds.), Human Physiology, 6th Ed., McGraw-Hill, New York, 1994, pp. 115–145.
26. C. Allen, Y. Yu, A. Eisenberg, and D. Maysinger. Cellular internalization of PCL20-b-PEO44 block copolymer micelles. Biochim. Biophys. Acta 1421:32–38 (1999).
27. R. Savic, L. Luo, A. Eisenberg, and D. Maysinger. Micellar nanocontainers distribute to defined cytoplasmic organelles. Science 300:615–618 (2003).
28. C. Vauthier, C. Dubernet, C. Chauvierre, I. Brigger, and P. Couvreur. Drug delivery to resistant tumors: the potential of poly(alkyl cyanoacrylate) nanoparticles. J. Control. Rel. 93:151–160 (2003).
29. C. Vauthier, C. Dubernet, E. Fattal, H. Pinto-Alphandary, and P. Couvreur. Poly(alkylcyanoacrylates) as biodegradable materials for biomedical applications. Adv. Drug Deliv. Rev. 55:519–548 (2003).
30. C. Cuvier, L. Roblot-Treupel, J. M. Millot, G. Lizard, S. Chevillard, M. Manfait, P. Couvreur, and M. F. Poupon. Doxorubicin-loaded nanospheres bypass tumor cell multidrug resistance. Biochem. Pharmacol. 44:509–517 (1992).
31. S. Bennis, C. Chapey, P. Couvreur, and J. Robert. Enhanced cytotoxicity of doxorubicin encapsulated in polyisohexylcyanoacrylate nanospheres against multidrug-resistant tumour cells in culture. Eur. J. Cancer 30A:89–93 (1994).
32. F. Nemati, C. Dubernet, A. Colin de Verdière, M. F. Poupon, L. Treupel Acar, F. Puisieux, and P. Couvreur. Some parameters influencing cytotoxicity of free doxorubicin loaded nanoparticles in sensitive and multidrug resistant leucemic murine cells: incubation time, number of particles per cell. Int. J. Pharm. 102:55–62 (1994).
33. A. Colin de Verdiere, C. Dubernet, F. Nemati, M. F. Poupon, F. Puisieux, and P. Couvreur. Uptake of doxorubicin from loaded nanoparticles in multidrug-resistant leukemic murine cells. Cancer Chemother. Pharmacol. 33:504–508 (1994).
34. A. Colin de Verdiere, C. Dubernet, F. Nemati, E. Soma, M. Appel, J. Ferte, S. Bernard, F. Puisieux, and P. Couvreur. Reversion of multidrug resistance with polyalkylcyanoacrylate nanoparticles: towards a mechanism of action. Br. J. Cancer 76:198–205 (1997).
35. X. Pepin, L. Attali, C. Domrault, S. Gallet, J. M. Metreau, Y. Reault, P. J. Cardot, M. Imalalen, C. Dubernet, E. Soma, and P. Couvreur. On the use of ion-pair chromatography to elucidate doxorubicin release mechanism from polyalkylcyanoacrylate nanoparticles at the cellular level. J. Chromatogr. B 702:181–191 (1997).
36. R. Mehta and T. G. Burke. Membrane biophysical parameters influencing anthracycline action. In W. Priebe (ed.), Anthracycline Antibiotics, ACS Symposium Series 574, American Chemical Society, Washington DC, 1995, pp. 222–240.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yi, Y., Kim, J., Kang, HW. et al. A Polymeric Nanoparticle Consisting of mPEG-PLA-Toco and PLMA-COONa as a Drug Carrier: Improvements in Cellular Uptake and Biodistribution. Pharm Res 22, 200–208 (2005). https://doi.org/10.1007/s11095-004-1187-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-004-1187-1