No Heading
Purpose.
The aim of this research was to develop a pH-dependent canine absorption model for studying pH effect on both dissolution in vitro and pharmacokinetics in vivo using the weak bases ketoconazole and dipyridamole as model drugs.
Methods.
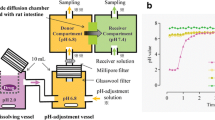
Ketoconazole and dipyridamole pH-dependent dissolution profiles in vitro were determined by dissolution test at different pH values using USP apparatus II and an Opt-Diss Fiber Optic UV System. In vivo absorption studies for ketoconazole and dipyridamole were performed with crossover design in three groups of beagle dogs under control (no treatment), pentagastrin, and famotidine treatments. Ketoconazole and dipyridamole plasma concentrations were quantified by gradient high performance liquid chromatography mass spectroscopy (HPLC MS/MS). Pharmacokinetic parameters were determined from individual plasma concentration vs. time profiles.
Results.
Ketoconazole and dipyridamole displayed pH-dependent dissolution. Increasing the pH of the dissolution medium from 1.2 to 6.8 reduced the extent of dissolution of ketoconazole and dipyridamole at 1 h by 96% and 92%, respectively. In vivo studies in dogs under control (no treatment), pentagastrin, and famotidine treatments show marked differences in systemic ketoconazole and dipyridamole exposure. Area under the concentration-time curve (AUC) increased more than 4-fold as compared to control group, whereas it increased nearly 30-fold for ketoconazole and 9-fold for dipyridamole with pentagastrin (gastric pH ∼2–3) as compared to famotidine (gastric pH ∼5–7.5) treatment.
Conclusions.
This work demonstrates a pH-dependent dissolution in vitro and absorption in vivo for the weak bases ketoconazole and dipyridamole independent of food effects. This model is useful to examine pH-dependent effects on oral drug absorption and for screening formulations to overcome the pH dependency.
Similar content being viewed by others
References
1. M. Akimoto, N. Nagahata, A. Furuya, K. Fukushima, S. Higuchi, and T. Suwa. Gastric pH profiles of beagle dogs and their use as an alternative to human testing. Eur. J. Pharm. Biopharm. 49:99–102 (2000).
2. C. Y. Lui, G. L. Amidon, R. R. Berardi, D. Fleisher, C. Youngberg, and J. B. Dressman. Comparison of gastrointestinal pH in dogs and humans: implications on the use of the beagle dog as a model for oral absorption in humans. J. Pharm. Sci. 75:271–274 (1986).
3. J. H. Lin. Species similarities and differences in pharmacokinetics. Drug Metab. Dispos. 23:1008–1021 (1995).
4. J. B. Dressman. Comparison of canine and human gastrointestinal physiology. Pharm. Res. 3:123–131 (1986).
5. G. P. Bodey. Fungal infections complicating acute leukemia. J. Chronic Dis. 19:667–687 (1966).
6. J. A. Como and W. E. Dismukes. Oral azole drugs as systemic antifungal therapy. N. Engl. J. Med. 330:263–272 (1994).
7. V. Fainstein, G. P. Bodey, L. Elting, A. Maksymiuk, M. Keating, and K. B. McCredie. Amphotericin B or ketoconazole therapy of fungal infections in neutropenic cancer patients. Antimicrob. Agents Chemother. 31:11–15 (1987).
8. S. C. Piscitelli, T. F. Goss, J. H. Wilton, D. T. D’Andrea, H. Goldstein, and J. J. Schentag. Effects of ranitidine and sucralfate on ketoconazole bioavailability. Antimicrob. Agents Chemother. 35:1765–1771 (1991).
9. USP–DI Drug Information for the Health Care Professional, Greenwood Village, United States Pharmacopeial Convention, Inc., 1991.
10. J. A. Carlson, H. J. Mann, and D. M. Canafax. Effect of pH on disintegration and dissolution of ketoconazole tablets. Am. J. Hosp. Pharm. 40:1334–1336 (1983).
11. W. O. Foye. Principles of Medicinal Chemistry, Lea and Febiger, Philadelphia, 1981.
12. R. A. Blum, D. T. D’Andrea, B. M. Florentino, J. H. Wilton, D. M. Hilligoss, M. J. Gardner, E. B. Henry, H. Goldstein, and J. J. Schentag. Increased gastric pH and the bioavailability of fluconazole and ketoconazole. Ann. Intern. Med. 114:755–757 (1991).
13. T. W. Chin, M. Loeb, and I. W. Fong. Effects of an acidic beverage (Coca-Cola) on absorption of ketoconazole. Antimicrob. Agents Chemother. 39:1671–1675 (1995).
14. P. Lelawongs, J. A. Barone, J. L. Colaizzi, A. T. Hsuan, W. Mechlinski, R. Legendre, and J. Guarnieri. Effect of food and gastric acidity on absorption of orally administered ketoconazole. Clin. Pharm. 7:228–235 (1988).
15. T. L. Russell, R. R. Berardi, J. L. Barnett, T. L. O’Sullivan, J. G. Wagner, and J. B. Dressman. pH-related changes in the absorption of dipyridamole in the elderly. Pharm. Res. 11:136–143 (1994).
16. G. L. Amidon, H. Lennernas, V. P. Shah, and J. R. Crison. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bio-availability. Pharm. Res. 12:413–420 (1995).
17. C. A. Knupp, W. C. Shyu, E. A. Morgenthien, J. S. Lee, and R. H. Barbhaiya. Biopharmaceutics of didanosine in humans and in a model for acid-labile drugs, the pentagastrin-pretreated dog. Pharm. Res. 10:1157–1164 (1993).
18. M. Goemann and T. G. Cantu. Ketoconazole and gastric acidity. Clin. Pharm. 12:802 (1993).
19. X. Lu, R. Lozano, and P. Shah. In situ dissolution testing using different uv fiber optic probes and instruments. Dissolution Technol. 10:6–15 (2003).
20. J. B. Dressman, G. L. Amidon, C. Reppas, and V. P. Shah. Dissolution testing as a prognostic tool for oral drug absorption: immediate release dosage forms. Pharm. Res. 15:11–22 (1998).
21. J. B. Dressman and C. Reppas. In vitro-in vivo correlations for lipophilic, poorly water-soluble drugs. Eur. J. Pharm. Sci. 11:S73–S80 (2000).
22. D. Lange, J. H. Pavao, J. Wu, and M. Klausner. Effect of a cola beverage on the bioavailability of itraconazole in the presence of H2 blockers. J. Clin. Pharmacol. 37:535–540 (1997).
23. D. C. Sadowski. Drug interactions with antacids. Mechanisms and clinical significance. Drug Saf. 11:395–407 (1994).
24. T. Zimmermann, R. A. Yeates, H. Laufen, G. Pfaff, and A. Wildfeuer. Influence of concomitant food intake on the oral absorption of two triazole antifungal agents, itraconazole and fluconazole. Eur. J. Clin. Pharmacol. 46:147–150 (1994).
25. N. Kohri, N. Miyata, M. Takahashi, H. Endo, K. Iseki, K. Miyazaki, S. Takechi, and A. Nomura. Evaluation of pH-independent sustained-release granules of dipyridamole by using gastric-acidity-controlled rabbits and human subjects. Int. J. Pharm. 81:49–58 (1992).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, R., Moench, P., Heran, C. et al. pH-Dependent Dissolution in Vitro and Absorption in Vivo of Weakly Basic Drugs: Development of a Canine Model. Pharm Res 22, 188–192 (2005). https://doi.org/10.1007/s11095-004-1185-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-004-1185-3