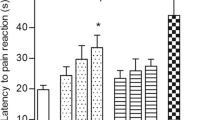
Comenic (5-hydroxy-4-oxo-4H-pyran-2-carboxylic) acid is regarded as a new analgesic agent. Comenic acid (CA) was studied in acute pain models using Wistar rats in the tail-flick and hot-plate tests. CA was administered in a single intramuscular injection 30 min before the tests. The reference drug (INN diclofenac) was administered at a dose of 6.6 mg/kg; the test drug (CA solution), at doses of 20, 40, and 60 mg/kg. Tests were performed 15 min and immediately before CA administration and 30, 45, and 60 min after administration. The maximum possible effect (MPE) was calculated for each test at the end of the studies. No statistically significant differences were found between experimental groups in the tail-flick test. The MPE in the hot-plate test for the test and reference drug groups was significantly higher than that in the control group at all time points (30, 45, and 60 min after administration of the drugs). CA was found to have a lesser effect on the formation of a spinal reflex (tail-flick test) but was rather effective in tests involving supraspinal structures (hot-plate test). No toxic manifestations (according to clinical observation and animal body weight) and dose dependence were identified.
Similar content being viewed by others
References
S. Bindu, S. Mazumder, and U. Bandyopadhyay, Biochem Pharmacol., 180, 1 – 21 (2020); https://doi.org/10.1016/j.bcp.2020.114147.
C. Phillips, E. Contreras, and J. Oswald, “NSAIDs, Opioids, and Beyond,” in: Pain Management – Practices, Novel Therapies and Bioactives, Intechopen (2021), Chap. 15; https://doi.org/10.5772/intechopen.93843.
L. A. Morrone, D. Scuteri, L. Rombola, et al., Curr. Neuropharmacol., 15(3), 444 – 456 (2017); https://doi.org/10.2174/1570159X14666161101092822.
B. V. Krylov, I. V. Rogachevskii, T. Shelykh, et al., New Nonopioid Analgesics: Understanding Molecular Mechanisms on the Basis of Patch-Clamp and Chemical Studies, Bentham Science Publishers Ltd., UAE (2017).
I. V. Rogachevskii, V. B. Plakhova, V. A. Penniyaynen, et al., Can. J. Physiol. Pharmacol., 100(1), 43 – 52 (2022); https://doi.org/10.1139/cjpp-2021-0286.
V. A. Penniyaynen, V. B. Plakhova, I. V. Rogachevskii, et al., Pathophysiology, 26(3-4), 245 – 252 (2019); https://doi.org/10.1016/j.pathophys.2019.06.003.
G. B. Mulder and K. Pritchett, Contemp. Top. Lab. Anim. Sci., 43(3), 54 – 55 (2004).
D. Le Bars, M. Gozariu, and S. W. Cadden, Pharmacol. Rev., 53(4), 597 – 652 (2001).
M. M. Morgan, J. H. Sohn, and J. C. Liebeskind, Brain Res., 502(1), 61 – 66 (1989); https://doi.org/10.1016/0006-8993(89)90461-7.
T. A. Bitkina and A. V. Basevich, Razrab. Regist. Lek. Sredstv, 10, 108 – 114 (2021); https://doi.org/10.33380/2305-2066-2021-10-4(1)-108-114.
A. V. Rybakova, M. N. Makarova, A. E. Kukharenko, et al., Vedomosti Nauchn. Tsentra Ekspert. Sredstv Med. Primen., 8(4), 207 – 217 (2018).
Instructions for medical use of Voltaren.
Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Guidance for Industry, U. S. DHHS, FDA, CDER (2005), p. 7.
A. N. Mironov (ed.), Handbook for Preclinical Drug Studies [in Russian], Grif i K, Moscow (2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 57, No. 11, pp. 20 – 23, November, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kovalenko, A.L., Petrov, A.Y., Lycheva, N.A. et al. Analgesic Effect of Comenic Acid in Acute Pain Models in Rats. Pharm Chem J 57, 1709–1711 (2024). https://doi.org/10.1007/s11094-024-03069-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-024-03069-2