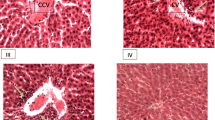
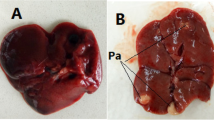
A liver capsule is a hepatoprotective polyherbal formulation that has been traditionally used for the treatment of various diseases, particularly liver disorders. The present study aimed to assess the safety of the liver capsule by investigating acute and subchronic toxicity in rats. Acute toxicity test was performed on rats that orally received a single dose of 500, 1000, 2000 mg/kg. In a subchronic toxicity assay, the animals received 300 and 600 mg/kg of the liver capsule for 45 consecutive days. All animals were daily evaluated for signs of toxicity and biochemical and hematological parameters and histopathological tissues examination were assessed at the end of the study. The liver capsule did not produce any mortality in the acute toxicity study, although a histopathological examination showed toxicity signs in the liver tissue. In the subchronic study, there was no mortality or changes in behavior, daily food and water intake; however, levels of alanine transaminase (ALT), lactate dehydrogenase (LDH), and Creatine kinase (CK) were increased and albumin and glucose levels decreased in treated groups. Also, liver and kidney tissues showed pathological changes in the treated animals. The LD50 value of the liver capsule is thought to be more than 2000 mg/kg. A low doseof the liver capsule (300 mg/kg) caused little change in biochemical parameters and histopathology of the liver tissue in a subchronic study. The employed dose was more than 10 times greater than the maximum human daily dose for liver disease treatment (20 – 30 mg/kg). Thus, it can be concluded that the recommended human dose of liver capsules is generally safe.





Similar content being viewed by others
References
D. Mans, Acad. J. Med. Plant., No. 1, 101–110 (2013).
M. Kamalinejad, J. M. Jafari, N. Nayebi, et al., J. Res. Med. Dent. Sci., No. 6, 494–500 (2018).
R. K. Dhiman, Y. K. Chawla, J. Gastroenterol. Hepatol., 50, 1807–1812 (2005).
S. Ansari, M. Siddiqui, F. Zaman, J. Res. Educ. Indian Med., 21, 101 – 105 (2017).
O. E. Kale, O. B. Akinpelu, A. A. Bakare, et al., BMC Complement Altern. Med., 18, 1–11 (2018).
S. Li, B. Zhang, N. Zhang, BMC Syst. Biol., No. 5, 1–13 (2011).
C. Girish, B. C. Koner, S. Jayanthi, et al., Indian J. Exp. Biol., 47, 257–263 (2009).
E. Ziaee, M. Razmjooei, E. Shad, M. H. Eskandari, LWT – Food Sci. Technol., 87, 406–412 (2018).
Q. H. Gao, C. S. Wu, M. Wang, J. Agric. Food Chem., 61, 3351–3363 (2013).
J. Chen, X. Liu, Z. Li, et al., J. Evidence-Based Complementary Altern. Med., 2017 (2017).
S. Kumar, A. Kamboj, A. K. Sharma, Asian J. Pharm. Pharmacol., No. 4, 238–244 (2018).
J.-bo Wang, H. Ping Zhao, Y. Ling Zhao, et al., PLoS One, 6 (2011); https://doi.org/10.1371/journal.pone.0024498.
H. R. Adhami, H. Farsam, L. Krenn, Phytother. Res., 25, 1148–1152 (2011).
M. Bahmani, N. Shahinfard, M. Rafieian-Kopaei, et al., J. Chem. Pharm. Sci., No. 8, 672–682 (2015).
A. Ahmadipour, F. Sharififar, M. Pournamdari, et al., Orient. Pharm. Exp. Med., 16, 287–293 (2016).
M. Iqbal, M. Butt, A. Shehzad, et al., Asian Pac. J. Trop. Med., 11, 209–213 (2018).
R. Z. Zhang, H. Qiu, N. Wang, F. L. Long, et al., Asian Pac. J. Trop. Med., No. 8, 841–847 (2015).
S. Saggu, M. I. Sakeran, N. Zidan, et al., Food Chem. Toxicol., 72, 138–146 (2014).
K. K. Chahal, R. Singh, A. Kumar, et al., Indian J. Nat. Prod. Resour., No. 8, 193–203 (2017).
I. E. Orhan, B. Şener, S. G. Musharraf, Exp. Toxicol. Pathol., 64, 205–209 (2012).
R. Ilavarasan, S. Mohideen, M. Vijayalakshmi, et al., Indian J. Pharm. Sci., 63, 504 (2001).
C. Bunchorntavakul, K. R. Reddy, Aliment. Pharmacol. Ther., 37, 3–17 (2013).
C. Calitz, L. Plessis, C. Gouws, et al., Expert Opin. Drug Metab. Toxicol., 11(10), 1551 – 1565 (2015).
L. Langmead, D. S. Rampton, Aliment. Pharmacol. Ther., 15, 1239–1252 (2001).
F. Altýnok-Yipel, İ. O. Tekeli, S. Y. Özsoy, et al., Int. J. Vitam. Nutr. Res., 90(3 – 4), 302 – 308 (2020).
H. M. Khan, S. Iqbal, Indian J. Pharm. Sci., 79(1), 124 – 130 (2017).
S. Kumar, A. Kamboj, A. K. Sharma, Asian J. Pharm. Pharmacol., No. 2, 238 – 244 (2018).
R. Mohebbati, M. Paseban, F. Beheshti, et al., J. Pharmacopuncture, 21(4), 249–257 (2018).
S. K. Nikolaevna, et al., Int. J. Innov. Pharm. Sci. & Res., 8(02), 1 – 8 (2020).
A. Rajopadhye, U. Anuradha, J. Evidence-Based Complementary Altern. Med., No. 2, 1 – 8 (2016).
M. Sayyah, N. Hadidi, M. Kamalinejad, J. Ethnopharmacol., 92(2 – 3), 325 – 329 (2004).
B. Ahmed, S. Khan, M. H. Masood, et al., J. Asian Nat. Prod. Res., No. 3, 218 – 223 (2008).
K. Bellassoued, H. Hamed, F. Ghrab, et al., Arch. Physiol. Biochem., No. 6, 486 – 496 (2021).
C. D. Klaassen, Casarett and Doull’s Toxicology - The Basic Science of Poisons, McGraw-Hill, New York (2013), vol. 12, pp. 29 – 32.
OECD (2001). Guidance Document on Acute Oral Toxicity.
F. K.-M. Chan, K. Moriwaki, M. De Rosa, in: Immune Homeostasis, Andrew L. Snow and Michael J. Lenardo (eds.), Springer, Totowa (2013), pp. 65 – 70.
K. Kotoh, M. Kato, M. Kohjima, et al., Exp. Ther. Med., No. 2, 195–199 (2011).
M. Vaubourdolle, O. Chazouilleres, R. Poupon, et al., Hepatology, 17, 423–428 (1993).
D. Patel, S. Desai, R. Devkar, et al., EXCLI J., 11, 566 (2012).
F. E. Uboh, M. I. Akpanabiatu, E. U. Eyong, et al., Acta Biol. Szeged., 49, 19–22 (2005).
H. R. Rasekh, P. Nazari, M. Kamli-Nejad, et al., J. Ethnopharmacol., 116, 21–26 (2008).
X. Wang, W. Zhang, Y. Wang, et al., Regul. Toxicol. Pharmacol., 58, 421–427 (2010).
I. A. Agyigra, J. I. Ejiofor, M. G. Magaji, Bull. Fac. Pharmacy, Cairo Univ., 55, 263–267 (2017).
S. Soyuncu, Y. Cete, A. E. Nokay, Clin. Toxicol., 46, 774–777 (2008).
J. Wang, Y. Zhao, X. Xiao, et al., J. Ethnopharmacol., 124, 18–25 (2009).
M. Yan, L. Y. Zhang, L. X. Sun, et al., J. Ethnopharmacol., 107, 308–311 (2006).
B. G. Ye, Y. Feng, S. Wang, Food Chem. Toxicol., 66, 278–285 (2014).
S. Sireeratawong, S. Vannasiri, U. Nanna, et al., ISRN Pharmacol., 2012, 1–7 (2012).
P. N. Pushparaj, H. K. Low, J. Manikandan, et al., J. Ethnopharmacol., 111, 430–434 (2007).
G. K. Singh, V. Kumar, J. Ethnopharmacol., 134, 992–995 (2011).
N. Ilaiyaraja, F. Khanum, Int. J. Pharm. Biol. Arch., No. 1, 155–163 (2010).
J. Solati, N. Soleimani, Acta Diabetol., 47, 3119–3127 (2010).
V. Chithra, S. Leelamma, Food Chem., 67, 229–231 (1999).
F. Fathiazad, S. Hamedeyazdan, M. K. Khosropanah, et al., Adv. Pharm. Bull., No. 3, 207–210 (2013).
M. Hemmati, S. Asghari, E. Zohoori, et al., Pak. J. Pharm. Sci., 28, 2095–2099 (2015).
N. N. Osman, E. J. Jambi, N. H. Aseri, Int. J. Pharm. Res. Sci., No. 6, 149–162 (2017).
P. Dhanapakiam, J. M. Joseph, V. K. Ramaswamy, et al., J. Environ. Biol., 29, 53 (2007).
A. Khoshvaghti, S. Nazifi, S. Derakhshaniyan, et al., J. Basic Appl. Sci., No. 8, 217–222 (2012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Monirvaghefi, A., Jeivad, F., Albooyeh, S. et al. Acute and Subchronic Oral Toxicity Study of a Hepatoprotective Polyherbal Formulation ‘Liver Capsule’ in Rats. Pharm Chem J 57, 1164–1173 (2023). https://doi.org/10.1007/s11094-024-03022-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-024-03022-3