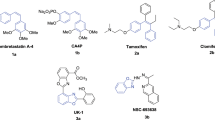
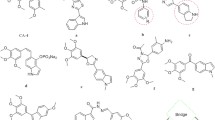
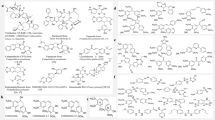
In the present work, a series of 1,2,4-triazole derivatives are designed as combretastatin A-4 analogs with the 4-nitrophenyl group and different aliphatic alkyl substituents, the designed compounds were synthesized and characterized by FT-IR, 1H NMR,13C NMR spectroscopy, and mass spectrometry. The synthesized compounds were tested as antiproliferative agents against human cancer laryngeal (Hep-2) cell line, the cytotoxicity of the synthesized compounds was evaluated by the treatment of a human normal kidney (Vero) cell line. The obtained results revealed that compound 5c, which has a n-propyl substituent, is the most active one and has lowest cytotoxicity against normal cells. This compound has the lowest IC50 value within the tested series; consequently, compound 5c could be considered a promising antiproliferative agent and a good candidate for further pharmacological studies. Molecular docking studies were implemented to determine the affinity of the synthesized compound toward the colchicine binding site.




Similar content being viewed by others
References
W. Shuai, G. Wang, Y. Zhang, Faqian, et al., J. Med. Chem., 64(12), 7963 – 7990 (2021).
G. R. Pettit, S. B. Singh, M. L. Niven, et al., J. Nat. Prod., No. 50, 119 – 131 (1987).
G. R. Pettit, S. B. Singh, E. Hamel, et al., Experientia, No. 45, 209 – 211(1989).
H.-Y. Guo, Z.-A. Chen, Q.-K. Shen, J. Enzyme Inhib. Med. Chem., 36(1), 1115 – 1144 (2021).
L. Lee, R. Davis, J. Vanderham, et al., Eur. J. Med. Chem., 43(9), 2011 – 2015 (2008).
G. C. Tron, T. Pirali, G. Sorba, et al., J. Med. Chem., No. 49, 3033 – 3044 (2006).
M. J. Peérez-Peérez, E. M. Priego, O. Bueno, et al., J. Med. Chem., 59(19), 8685 – 8711 (2016).
R. Romagnoli, P. G. Baraldi, O. Cruz-Lopez, et al., J. Med. Chem., 53(10), 4248 – 4258 (2010).
M. Metzler, H. Neumann, Xenobiotica, 7(3), 117 – 132 (1977).
G. Valdomir, M. de los Angeles Fernandez, I. Lagunes, et al., New J. Chem., 42(16), 13784 – 13789 (2018).
H. Kommidi, H. Guo, F. Nurili, et al., J. Med. Chem., 61(9), 4256–4262 (2018).
J. Akhtar, A. A. Khan, Z. Ali, et al., Eur. J. Med. Chem., No. 125, 143 – 189 (2017).
Y. W. He, C. Z. Dong, J. Y. Zhao, et al., Eur. J. Med. Chem., No. 76, 245 – 55 (2014).
J. J. Garcýa-Vanegas, A. Ramírez-Villalva, A. Fuentes-Benites, et al., J. Chem. Sci., 131(4), 1 – 10 (2019).
C. Satheeshkumar,M. Ravivarma, P. Arjun, et al., J. Chem. Sci., 127(3), 565 – 574 (2015).
S. M. M. Lopes, J. S. Novais, D. C. S. Costa, et al., Eur. J. Med. Chem., No. 143, 1010 – 1020 (2018).
M. F. Mady, G. E. Awad, K. B. Jorgensen, Eur. J. Med. Chem., No. 84, 433 – 443 (2014).
K. Rajavelu, M. Subaraja, P. Rajakumar, New J. Chem., 42(5), 3282 – 3292 (2018).
K. A. Kumar, B. Kalluraya, S. M. Kumar, Phosphorus Sulfur Silicon Relat. Elem., 193(5), 294 – 299 (2018).
Y. Chinthala, S. Thakur, S. Tirunagari, et al., Eur. J. Med. Chem., No. 93, 564 – 573 (2015).
J. H. Xu, Y. L. Fan, J. Zhou, J. Heterocyclic Chem., 55(8), 1854 – 1862 (2018).
B. Khan, A. Naiyer, F. Athar, et al., J. Biomol. Struct. Dyn., 39(2), 457 – 475 (2021).
Y. H. Li, B. Zhang, H. K. Yang, et al., Eur. J. Med. Chem., No. 125, 1098 – 1106 (2017).
S. Al-Mansury, A. A. Balakit, F. F. Alkazazz, et al., Pharm. Chem. J., 55(6), 556 – 565 (2021).
R. I. Freshney, Culture of Animal Cells: A Manual of Basic Technique (5thed.), John Wiley & Sons, Inc., USA (2005), pp. 252 – 253.
L. Pinzi, G. Rastelli, Int. J. Mol. Sci., 20(18), 4331 (2019).
D. N. do Amaral, B. C. Cavalcanti, D. P. Bezerra, et al., PloS One, 9(3), e85380 – e85380 (2014).
M. Hagras, M. A. el Deeb, H. S. A. Elzahabi, E. B. Elkaeed, J. Enzyme Inhib. Med. Chem., 36(1), 640 – 658 (2021).
A. E. Prota, F. Danel, F. Bachmann, K. Bargsten, et., J. Mol. Biol., 426(8), 1848 – 1860 (2014).
E. Kellenberger, J. Rodrigo, P. Muller, et al., Proteins: Struct. Funct. Genet., 57(2), 225 – 242 (2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Balakit, A.A., Abu-El-Halawa, R., Alsadoon, A.H. et al. Synthesis, Antiproliferative, and Molecular Docking Studies of 3-Mercapto-1,2,4-Triazole Derivatives as Combretastatin A-4 Analogs. Pharm Chem J 57, 1001–1007 (2023). https://doi.org/10.1007/s11094-023-02977-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-023-02977-z