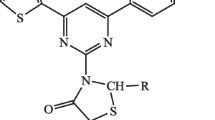
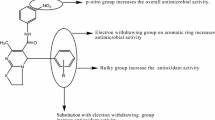
Thiazolidinediones (TZDs) are the vital component of a number of pharmaceuticals and biologically active compounds. Owing to the diversified pharmaceutical applications of TZD derivatives, we conceived a new synthetic approach for the preparation of a series of spiro-thiazolidinediones by the reaction of synthesized TZDs with thioglycolic acid. Melting point and thin-layer chromatography ascertained the purity of the synthesized compounds. Elemental analysis, FTIR, 1H and 13C NMR spectral studies have been used for the characterization of the synthesized compounds. The synthesized chalcones, thiazine, TZDs, and spiro-thiazolidinediones are in accordance with the standard values of different spectral techniques. The appearance of a sharp singlet of two protons of CH2 at 2.5 – 3.0 ppm in 1H NMR of spiro-thiazolidinediones, which was absent in TZDs, suggests the formation of a spiro ring. All the novel compounds were screened for their antimicrobial activity on Gram-negative (P. aeruginosa, E. coli) and Gram-positive (B. subtilis, S. aureus) bacteria. The final synthesized compounds exhibited excellent to good responses against the tested microbes. The change of the substituent and its position plays a significant role in the activity of the synthesized spiro-thiazolidinediones.
Similar content being viewed by others
References
H. A. Soleiman, Open Catal. J., 4, 18 – 26 (2011).
G. Shukla, A. K. Tiwari, V. K. Singh, et al., Chem. Biol. Drug. Des., 72(6), 533 – 539 (2008).
V. Srivastava, A. M. Srivastava, A. K. Tiwari, et al., Chem. Biol. Drug. Des., 74(3), 297 – 301 (2009).
A. K. Tiwari, A. K. Mishra, A. Bajpai, et al., Bioorg. Med. Chem. Lett., 16(17), 4581 – 4585 (2006).
E. A. Deniz, O. H. A. Mehtap, A. Oðuzhan, et al., Synth. Commun., 48(19), 2510 – 2521 (2018).
S. Aggarwal, D. Sinha, A. K. Tiwari, et al., Spectrochim. Acta. A Mol. Biomol. Spectrosc., 143, 309 – 318 (2015).
A. L. Acosta, and A. D. Rodríguez, J. Nat. Prod., 55(7), 1007 – 1012 (1992).
M. Sannigrahi, Tetrahedron, 55, 9007 – 9071 (1999).
M. M. Youssef and M. A. Amin, Molecules, 15(12), 8827 – 8840 (2010).
R. M. Shaker, Y. R. Ibrahim, F. F. Abdel-Latif, et al., Arkivoc., 2, 57 – 68 (2011).
Y. W. Chin, A. A. Salim, B. N. Su, et al., J. Nat. Prod., 71(3), 390 – 395 (2008).
W. L. Wang, T. J. Zhu, H. W. Tao, et al., Chem. Biodivers., 4(12), 2913 – 2919 (2007).
Varun, Sonam, and R. Kakkar, Med. Chem. Comm., 10(3), 351 – 368 (2019).
R. P. Chinnasamy and R. Sundararajan, J. Saudi Chem. Soc., 17(3), 337 – 343 (2016).
D. W. Kim, M. J. Curtis-Long, H. J. Yuk, et al., Food Chem., 153, 20 – 27 (2014).
X. B. Chen, H. Y. Zhu, and K. Bao, Acta Pharmacol. Sin., 42, 1160 – 1170 (2021).
R. Nath, S. Pathania, G. Grover, et al., J. Mol. Str., 1222, 128900 (2020).
R. E. Ferraz de Paiva, E. G. Vieira, D. Rodrigues da Silva, et al., Front. Mol. Biosci., 7, 627272 (2021).
Y. Z. Zhang, H. Z. Du, H. J. Liu, et al., Arch. Pharm., 353(3), e1900299 (2020).
Y. Ding, L. Zhao, Y. Fu, et al., Molecules, 26(1), 176 (2020).
M. Tugrak, H. I. Gul, K. Bandow, et al., Bioorg. Chem., 90, 103095 (2019).
B. Salehi, C. Quispe, I. Chamkhi, et al., Front. Pharmacol., 11, 592654 (2021).
S. Narwal, S. Kumar, and P. K. Verma, Res. Chem. Intermed., 47, 1625 – 1641 (2021).
H. A. Jasim, L. Nahar, M. A. Jasim, et al., Biomolecules, 11(8), 1203 (2021).
S. L. Gaonkar and U. N. Vignesh, Res. Chem. Intermed., 43, 6043 – 6077 (2017).
S. Farooq, Z. Ngaini, and N. A. Mortadza, Bull. Korean Chem. Soc., 41, 918 – 924 (2020).
A. Rani, A. Anand, K. Kumar, et al., Expert. Opin. Drug Discov., 14(3), 249 – 288 (2019)
N. Agrawal, Curr. Chem. Lett., 119 – 138 (2021).
I. Mishra, R. Mishra, S. Mujwar, et al., J. Heterocycl. Chem., 57, 2304 – 2329 (2020).
A. Vaidya, D. Pathak, and K. Shah, Chem. Biol. Drug Des., 97, 572 – 591 (2021).
P. P. Singh, S. Bansal, K. Rawat, et al., Res. J. Chem. Environ., 25(5) (2021).
P. P. Singh, M. Kumar and J. K. Ajish, Res. J. Chem. Environ., 25(11), 48 (2021)
K. El-Adl, H. Sakr, S. S. A. El-Hddad, et al., Arch. Pharm., 354(7), e2000491 (2021).
B. Sever, M. D. Altýntop, Y. Demir, et al., Open Chem., 19(1), 347–357 (2021).
M. Oguchi, K. Wada, H. Honma, et al., J. Med. Chem., 43(16), 3052 – 3066 (2000).
Sucheta, S. Tahlan, and P. K. Verma, Chem. Cent. J., 11(1), 130 (2017).
N. Long, A. Le Gresley, and S. P. Wren, Chem. Med. Chem., 16(11), 1716 – 1735 (2021).
H. E. Lebovitz, Curr. Diab. Rep., 19(12), 151 (2019).
Z. Huiying, C. Guangying, and Z. Shiyang, J. Enzyme Inhib. Med. Chem., 34(1), 981–989 (2019).
S. Kotha, G. Sreevani, L. U. Dzheileva, et al., Beilstein J. Org. Chem., 15, 2774 – 2781 (2019).
K. Dhara, S. Paladhi, G. C. Midya, et al., Org. Biomol. Chem., 9(10), 3801 – 3807 (2011).
C. B. Apaydýn, M. Tansuyu, Z. Cesur, et al., Bioorg. Chem., 112, 104958 (2021).
J. Sun, L. L. Zhang, E. Y. Xia, et al., J. Org. Chem., 74(9), 3398 – 3401 (2009).
V. S. Rao, S. V. S. Gupta, P. Giridhar, et al., Ind. J. Heterocycl. Chem., 9, 247 – 250 (2000).
W. L. Yang, F. F. Tang, F. S. He, et al., Org. Lett., 17, 4822?4825 (2015).
A. Srivastava, N. Srivastava, and U. N. Tripathi, Bull. Chem. Soc. Ethiop., 35(1), 61 – 76 (2021).
A. Srivastava, N. Srivastava, U. N. Tripathi, et al., Chem. Chem. Technol., 13(1), 23 – 32 (2019).
U. H. Shah, and S. G. Patel, Asian J. Pharm. Clin. Res., 10(2), 403 – 406 (2017).
C. Mahon and D. Lehman, Textbook of Diagnostic Microbiology, 6 (2018).
J. Howard, and M. D. Balbi, Pediatr. Rev., 25(8), 284 – 288 (2004).
A. Sirogianni, G. G. Kournoutou, A. Bougas, et al., Antibiotics (Basel), 10(4), 394 (2021).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Srivastava, K., Singh, R.B., Srivastava, A. et al. Synthesis, Characterization, and Antimicrobial Evaluation of Some Novel 3′-(6-(Substituted Phenyl)-4-Phenyl-6H-1,3-Thiazine-2-yl)Spiro[Indoline-3,3′-Isothiazolidine]-2,4′-Dione Derivatives. Pharm Chem J 57, 992–1000 (2023). https://doi.org/10.1007/s11094-023-02976-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-023-02976-0