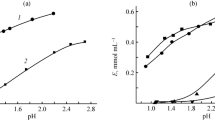
Results of screening studies of the sorption of naturally occurring biologically active compounds (phenolcarboxylic acids, coumarins, flavonoids, and alkaloids) on aluminum- and iron-containing metal-organic frameworks (MOFs) are presented. The structures of the synthesized MOFs were characterized by powder x-ray diffraction, porosimetry, and ATR-FTIR spectroscopy. Sorption of the analytes was quantitatively analyzed by UV spectrophotometry. The sorption activity of iron-containing MOFs was determined to be higher than that of aluminum-containing MOFs. The difference in sorption capacities indicates that targeted selection of MOFs for selective retention or separation of a mixture of chemically different substances is possible.






Similar content being viewed by others
References
C. X. Yang and X.-P. Yan, Chin. J. Anal. Chem., 41, 1297 – 1300 (2013); 10.1016 / S1872-2040(13)60677-5.
H. M. Perez-Cejuela, J. M. Herrero-Martinez, and E. F. Simo-Alfonso, Molecules, 25(18), 4216 (2020); https://doi.org/10.3390/molecules25184216.
X. Li, W. Ma, H. Li, et al., Coord. Chem. Rev., 397, 1 – 13 (2019); 10.1016 / j.ccr.2019.06.014.
X. Wang and N. Ye, Electrophoresis, 38(24), 3059 – 3078 (2017); 10.1002 / elps.201700248.
Z. Moradi, E. A. Dil, and A. Asfaram, Analyst, 144(14), 4351 – 4361 (2019); 10.1039 / C9AN00120D.
M. Zhang, H. Liu, Y. Han, et al., J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 1159, 122343 (2020); https://doi.org/10.1016/j.jchromb.2020.122343.
X. Pang, H. Liu, H. Yu, et al., J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 1125, 121715 (2019); https://doi.org/10.1016/j.jchromb.2019.121715.
A. Yohannes and S. Yao, Analyst, 144(23), 6989 – 7000 (2019); https://doi.org/10.1039/C9AN01362H.
P. Chen, X. Li, X. Yan, and M. Tian, Separations, 8(2), 22 (2021); https://doi.org/10.3390/separations8020022.
Ali K. Attia, M. M. Abdel-Moety, and S. G. Abdel-Hamid, New J. Chem., 41(18), 10189 – 10197 (2017); https://doi.org/10.1039/C7NJ01679D.
R. Ou, H. Zhang, J. Wei, et al., Adv. Mater., 30(34), 1802767 (2018); https://doi.org/10.1002/adma.201802767.
S. Selvaraj, S. Krishnaswamy, V. Devashya, et al., Med. Res. Rev., 34(4), 677 – 702 (2014); https://doi.org/10.1002/med.21301.
M. Khater, D. Ravishankar, F. Greco, and H. M. Osborn, Future Med. Chem., 11(21) (2019); https://doi.org/10.4155/fmc-2019-0237.
B. V. de Voorde, B. Bueken, J. Denayer, et al., Chem. Soc. Rev., 43(16), 5766 – 5788 (2014); https://doi.org/10.1039/C4CS00006D.
B. Liu, F. Yang, Y. Zou, and Y. Peng, J. Chem. Eng. Data, 59(5), 1476 – 1482 (2014); https://doi.org/10.1021/je4010239.
G. Awasthi, S. Shivgotra, S. Nikhar, et al., Polymers (Basel), 14(21), 4710 (2022); https://doi.org/10.3390/polym14214710.
J. Wang, J. Wan, Y. Ma, et al., RSC Adv., 6(113), 112502 – 1125011 (2016); https://doi.org/10.1039/C6RA24429G.
T. Loiseau, C. Serre, C. Huguenard, et al., Chem. Eur. J., 10, No. 6, 1373 – 1382 (2004); https://doi.org/10.1002/chem.200305413.
S. Chen, X. Song, J. Lyu, et al., J. Am. Chem. Soc., 142(9), 4419 – 4428 (2020); https://doi.org/10.1021/jacs.9b13414.
S. Chen, S. Mukherjee, B. E. G. Lucier, et al., J. Am. Chem. Soc., 141(36), 14257 – 14271 (2019); https://doi.org/10.1021/jacs.9b06194.
CAS 1416330-84-1 CAU-10 – Organic Frame Material, Alfa Chemistry; https: //mof.alfa-chemistry.com/product/cau-10-cas-1416330-84-1-299553.html?nid= (accessed Sept. 18, 2022).
P. D. Du, H. T. M. Thanh, T. C. To, et al., J. Nanomater., 2019, e6061275 (2019); https://doi.org/10.1155/2019/6061275.
Y.-Y. Cui, C.-X. Yang, X.-D. Yang, and X.-P. Yan, J. Chromatogr. A, 1544, 8 – 15 (2018); https://doi.org/10.1016/j.chroma.2018.02.046.
Q. Liu, J. Gao, Z. Zheng, et al., Talanta, 203, 248 – 254; 10.1016 / j.talanta.2019.05.073.
S. E. Miller, M. H. Teplensky, P. Z. Moghadam, and D. Fairen-Jimenez, Interface Focus, 6(4), 20160027 (2016); https://doi.org/10.1098/rsfs.2016.0027.
C. Volkringer, T. Loiseau, N. Guillou, et al., Inorg. Chem., 49(21), 9852 – 9862 (2010); https://doi.org/10.1021/ic101128w.
A. Samokhvalov, Chem. Eur. J., 21(47), 16726 – 16742 (2015); https://doi.org/10.1002/chem.201502317.
A. L. Nuzhdin, K. A. Kovalenko, D. N. Dybtsev, and G. A. Bukhtiyarova, Mendeleev Commun., 20(1), 57 – 58 (2010); https://doi.org/10.1016/j.mencom.2010.01.022.
A. R. Fakhari, S. Asadi, H. M. Kosalar, et al., Anal. Methods, 9(38), 5646 – 5652 (2017); https://doi.org/10.1039/C7AY01093A.
C. Qi, Q. Cai, P. Zhao, et al., J. Chromatogr. A, 1449, 30 – 38 (2016); https://doi.org/10.1016/j.chroma.2016.04.055.
A. Sigel and H. Sigel, Met.-Based Drugs, 5(5), 262 (1998); https://doi.org/10.1155/MBD.1998.262a.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 57, No. 5, pp. 30 – 37, May, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shaykhutdinov, I.H., Ryazanova, T.K., Sokolova, I.V. et al. Sorption of Some Biologically Active Compounds of Plant Origin on Metal-Organic Frameworks. Pharm Chem J 57, 675–682 (2023). https://doi.org/10.1007/s11094-023-02937-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-023-02937-7