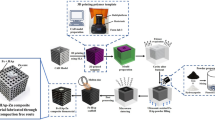
The purpose of this study was to create biodegradable composites using a synthetic polymer of polylactic-co-glycolic acid (PLGA), in which gentamicin or gentamicin and hydroxyapatite were included in order to achieve a local prolonged release of the drug in orthopedic disorders. The physico-chemical characterization of the biomaterial was achieved by Fourier-transform infrared spectroscopy (FT-IR), HPLC with diode array detector (DAD), and scanning electron microscopy (SEM). To determine whether the antibiotic inclusion in biomaterials alters its antibacterial activity, several studies were conducted on pathogenic bacteria present in bone infections (Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumonia, and Escherichia coli). SEM analysis showed that the surface of PLGA microspheres was modified to become slightly rough with submicron pores, which would favor the gentamicin release. Particle size distribution studied by DLS in PLGA-gentamicin and PLGA-HA-gentamicin presents small particles with diameters up to 450 nm. By HPLC-DAD we find out that the drug loading was of 13.26% (PLGA-gentamicin) and 7.28% (PLGA-HA-Gentamicin). We have also tracked the release profile of gentamicin included in the material, finding that the antibiotic release was improved by encapsulating it in delivery systems, contributing to sustained release for a long period of time (about 30 days). The biomaterials had antibacterial action on all tested microbial strains except on Pseudomonas aeruginosa.







Similar content being viewed by others
References
S. Xiaoyu, X. Chun, W. Gang, et al., Polymers, 9, 189 – 207 (2017). doi:https://doi.org/10.3390/polym9060189.
K. Sujit, S. Debnath, A. Saisivam, and O. Wahab, J. Pharmaceut. Biomed., 145, 854 – 859 (2017). https://doi.org/10.1016/j.jpba.
Y. Mo and L. Y. Lim, J. Control. Release, 108, 244 – 262 (2005). doi:https://doi.org/10.1016/j.jconrel.2005.08.013.
H. Gu, C. Song, D. Long, et al., Polym. Int., 56, 1272 – 1280 (2007). doi:https://doi.org/10.1002/pi.2272.
J. Neamþu, M. V. Bubulicã, A. Rotaru, et al., J. Therm. Anal. Calorim., 127, 1567 – 1582 (2017). doi:https://doi.org/10.1007/s10973-016-5905-9.
M. V. Ciocîlteu, A. G. Mocanu, A. Mocanu, et al., Acta Pharm., 68, 129 – 144 (2018). doi:https://doi.org/10.2478/acph-2018-0011.
A. G. Mocanu, A. Turcu-Þtiolicã, I. Belu, et al., Rev. Chim., 69, 1132 – 1138 (2018).
M. V. Ciocîlteu, P. Podgoreanu, C. Delcaru, et al., Farmacia, 67, 580 – 586 (2019). doi:https://doi.org/10.31925/farmacia.2019.4.4.
T. J. Levingstone, S. Herbaj, and N. J. Dunne, Nanomaterials, 9, 1570 – 1591 (2019). doi:https://doi.org/10.3390/nano9111570.
J. M. Fritz and J. R. McDonald, Phys. Sportsmed., 36, 50 – 54 (2008). doi:https://doi.org/10.3810/psm.2008.12.11.
A. Gyselynck, A. Forrey, and R. Cutler, J. Infect. Dis., 124, 70 – 76 (1971). doi:https://doi.org/10.1093/infdis/124.supplement1.s70.
A. Turcu-Þtiolicã, M. V. Bubulicã, O. E. Nicolaescu, et al., Rev. Chim., 69, 1944 – 1948 (2018).
O. Ionescu, M. V. Ciocîlteu, C. V. Manda, et al., Mater. Plast., 56, 534 – 537 (2019).
H. Nojehdehian, M. Ekrami, M. Shahriari, et al., Biomed. Res., 27, 70 – 78 (2016).
E. M. Hetrick and M. H. Schoenfisch, Chem. Soc. Rev., 35, 780–789 (2006). doi:https://doi.org/10.1039/b515219b.
S. B. Goodman, Z. Yao, M. Keeney, and F. Yang, Biomaterials, 34, 3174 – 3183 (2013). doi:https://doi.org/10.1016/j.biomaterials.2013.01.074.
I. G. Kang, C. I. Park, H. Lee, et al., Materials, 11, 258 – 271 (2018). doi:https://doi.org/10.3390/ma11020258.
C. Wischke and S. P. Schwendeman, Int. J. Pharm., 364, 298 – 327 (2008). doi:https://doi.org/10.1016/j.ijpharm.2008.04.042.
F. A. Muller, L. Muller, I. Hofmann, et al., Biomaterials, 27, 3955 – 3963 (2006). doi:https://doi.org/10.1016/j.biomaterials.2006.02.031.
D. Lickorish, J. A. Ramshaw, J. A. Werkmeister, et al., J. Biomed. Mater. Res. A, 68, 19 – 27 (2004). doi:https://doi.org/10.1002/jbm.a.20031.
X. M. Liu, Y. Zhang, F. Chen, et al., Pharm. Res., 29, 3169 – 3179 (2012). doi:https://doi.org/10.1007/s11095-012-0812-7.
C. Makarov, V. Cohen, A. Raz-Pasteur, and I. Gotman, Eur. J. Pharm. Sci., 62, 49 – 56 (2014). doi:https://doi.org/10.1016/j.ejps.2014.05.008.
D. P. Lew and F. A. Waldvogel, Lancet, 364, 369 – 379 (2004). doi:https://doi.org/10.1016/S0140-6736(04)16727-5.
E. García del Pozo, J. Collazos, J. A. Cartón, et al., Rev. Esp. Quimioter., 31, 217 – 225 (2018).
Z. Q. He and L. Z. Xiong, J. Macromol. Sci. B, 49, 66 – 74 (2010). doi:https://doi.org/10.1080/00222340903343731.
F. Ramazani, W. Chen, C. F. van Nostrum, et al., Int. J. Pharm., 499, 358 – 367 (2016). doi:https://doi.org/10.1016/j.ijpharm.2016.01.020.
Y. Yeo and K. Park, Arch. Pharm. Res., 27, 1 – 12 (2004). doi:https://doi.org/10.1007/BF02980037.
M. C. Chuong, J. Chin, J. W. Han, et al., Int. J. Pharmaceut. Anal., 4, 25 – 29 (2013).
M. Lungu, V. Tsakiris, and E. Enescu, Acta Phys. Pol. A, 125, 327 – 330 (2014). doi:https://doi.org/10.12693/APhysPolA.125.327.
M. Kaszuba, D. McKnight, M. T. Connah, and F. K. McNeil-Watson, J. Nanopart. Res., 10, 823 – 829 (2008). doi:https://doi.org/10.1007/s11051-007-9317-4.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Turcu-Ştiolică, A., Ciocîlteu, M.V., Podgoreanu, P. et al. PLGA-Gentamicin and PLGA-Hydroxyapatite-Gentamicin Microspheres for Medical Applications. Pharm Chem J 56, 645–653 (2022). https://doi.org/10.1007/s11094-022-02689-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-022-02689-w