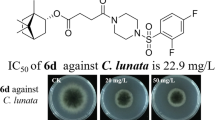
Phloroglucinols of Dryopteris fragrans (L.) Schott plant show strong antifungal activity. In previous work, we isolated a wide range of phloroglucinols from the plant and found that pseudoaspidinol of D. fragrans and its structural modifications (methylphloroglucinol derivatives) showed stronger antifungal activity and confirmed that the butyryl group of these compounds accounted for this activity. In this study, we designed a series of phloroglucinol derivatives (A1–A9) and methylphloroglucinol derivatives (B1–B9) by introducing acyl groups with diverse carbon numbers in the C-2 position, synthesized these compounds, and evaluated the anti-dermatophyte activity of compounds A1–A5 and B1–B5 on Trichophyton rubrum and Trichophyton mentagrophytes fungal species. The results showed that compound A5 exhibited the strongest activity. These compounds can be used as leads for the development of new antifungal agents.


Similar content being viewed by others
References
M. Ruhnke, V. Rickerts, O. A. Cornely, et al., Mycoses, 54, 279 – 310 (2011).
L. E. Cowen, D. Sanglard, S. J. Howard, et al., Cold Spring Harbor Perspect. Med., 5, a019752 (2014).
L. Alcazar-Fuoli and E. Mellado, Br. J. Haematol., 166, 471 – 84 (2014).
N. M. Revie, K. R. Iyer, N. R. Robbins, et al., Curr. Opin. Microbiol., 45, 70 – 76 (2018).
H. Su, L. Han, and X. Huang, J Antibiot, 71, 978 – 91 (2018).
D. G. Sant, S. G. Tupe, C. V. Ramana, et al., J. Appl. Microbiol., 121, 1498 – 1510 (2016).
A. M. Fuentefria, B. Pippi, D. F. Dalla Lana, et al., Lett. Appl. Microbiol., 66, 2 – 13 (2018).
D. S. Perlin, R. Rautemaa-Richardson, and A. Alastruey-Izquierdo, Lancet Infect. Dis., 17, e383 - e92 (2017).
S. K. Shrestha, A. Garzan, and S. Garneau-Tsodikova, Eur. J. Med. Chem., 133, 309 – 18 (2017).
M. Z. Zhang, C. Y. Jia, Y. C. Gu, et al., Eur. J. Med. Chem., 126, 669 – 74 (2017).
Y. H. Huang, W. M. Zeng, G. Y. Li, et al., Molecules, 19, 507 – 13 (2014).
X. Liu, J. Liu, T. Jiang, et al., J. Ethnopharmacol., 226, 36 – 43 (2018).
H. Kuang, C. Sun, Y. Zhang, et al., Fitoterapia, 80, 134 – 137 (2009).
S. Emami, S. Shojapour, M. A. Faramarzi, et al., Eur. J. Med. Chem., 66, 480 – 488 (2013).
V. B. Urlacher and M. Girhard, Trends Biotechnol., 37, 882 – 897 (2019).
H. Q. Fan, Z. B. Shen, Y. F. Chen, et al., J. Chin. Med. Mater., 35, 1981 – 1985 (2012).
Y. Sun, F. Mu, C. Li, et al., Chem.-Biol. Interact., 204, 88 – 97 (2013).
N. Li, C. Gao, X. Peng, et al., Res. Microbiol., 165, 263 – 72 (2014).
Y. Xu, G. R. Pang, D. Q. Chin. J. Ophthalmol., 46, 38 – 42 (2010).
O. Simonetti, C. Silvestri, D. Arzeni, et al., Mycoses, 57, 233 – 239 (2014).
J. M. Muhlbacher. Clin Dermatol, 9(4), 479 – 485 (1991).
X. J. Li, Y. J. Fu, M. Luo, et al., J. Pharm. Biomed. Anal., 61, 199 – 206 (2012).
Conflict of Interest
The authors declare that they have no conflicts of interest.
Funding
The work was supported by Special Innovation Project of Guangdong Education Department (Natural Science) (2017KTSCX107, 2020KZDZX1131), Science and Technology Planning Project of Guangdong Province (2014A020212602, 2017ZC0199), National Innovation and Entrepreneurship Training Program for College Students (201910573010), Medical Scientific Research Foundation of Guangdong Province (B2019004), and the Innovation and Strengthening Project of Guangdong Pharmaceutical University ‘Guangdong Province Graduate Education Innovation Program in 2020’.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, M., Lai, W., Li, J. et al. Design, Synthesis and Antifungal Activity of Phloroglucinol Derivatives. Pharm Chem J 56, 356–360 (2022). https://doi.org/10.1007/s11094-022-02651-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-022-02651-w