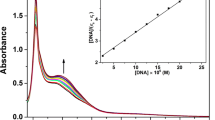
This study was aimed to synthesize and characterize two new copper(II) complexes, [Cu(phen)(Br-impi)]2+ (1) and [Cu(phen)(M-impi)]2+ (2) (where phen is 1,10-phenanthroline; Br-impi is 2-(2-(4-bromophenyl)-4- (pyridin-2-yl)-4,5-dihydro-1H-imidazol-5-yl)pyridine, andM-impi is 2-(2-(4-methoxyphenyl)-4-(pyridin- 2-yl)-4,5-dihydro-1H-imidazol-5-yl)pyridine), and to study their DNAbinding activity and cytotoxic activity. The interaction of complexes with ds-DNA has been investigated by spectroscopic methods, viscosity measurements, and agarose gel electrophoresis. The results indicate that complex 1 can bind to ds-DNA better than doescomplex 2 with binding constants 1.9 × 105M-1 and 3.9 × 104M-1, respectively.According to the UV titration method, complex 1 is capable of extractingethidium bromide molecules from DNA base pairs at lower concentrations than complex 2. In oxidative medium, both complexes produce complete degradationof plasmid DNA. Thecomplexes were screened against five human cell lines, namely A 549, PC-3, BEAS-2B, HCT-116 and MDZ-MB-231, and it was found that copper(II) complexes of trisubstituted imidazoleexhibited cytotoxic activity.










Similar content being viewed by others
References
B. Rosenberg, L. Vancamp, and T Krigas, Nature, 205, 698 – 699 (1965).
S. Chandra and R. Kumar, Trans. Metal Chem., 29(3), 269 – 275 (2004).
S. Chandra, R. Gupta, N. Gupta, and S. S. Bawa, Trans. Metal Chem., 31(2), 147 – 151 (2006).
C. Gurudevaru, M. Gopalakrishnan, H. Hemachandran, et al., Appl. Organomet. Chem., (2018).
R. Uauy, M. Olivarez, and M. Gonzalez, Am. J. Clin. Nutr., 67, 952S – 959S (1998).
K. D. Daniel, P. Gupta, R. H. Harbach, et al., Biochem. Pharmacol., 67(6), 1139 – 1151 (2004).
H. Xie and Y. J. Kank, Curr. Med. Chem., 16(10), 1304 – 1314 (2009).
Z. Xiao, P. S. Donnelli, M. Zimmermann, and A. G. Wedd, Inorg. Chem., 47(10), 4338 – 4347 (2008).
A. Gupte and R. J. Mumper, Cancer Treat. Rev., 35(1), 32 – 46 (2009).
V. Gandin, M. Porshia, F. Tisado, et al., J. Med. Chem., 51(4), 798 – 808 (2009).
E. D. Clercq, M. Cools, J. Balzarini, et al., Antimicrob. Agents Chemother., 35(4), 679 – 684 (1991).
A. M. G. Mawwar, N. M. Grant, Randa, and S. A. M. Elseginy, Der Pharma Chemica, 5(1), 241 – 255 (2013).
S. P. Zala, R. Badmanaban, D. J. Sen, and C. N. Patel, J. Appl. Pharm. Sci., 2(07), 202 – 208 (2012).
F. Hadizadeh, H. Hosseinzadeh, V. S. MotamedShariaty, et al., Iran. J. Pharm. Res., 7(1), 29 – 33 (2008).
S. G. Dandale, A. S. Sonar, and P.R. Solanki, Int. J. Chem. Environ. Pharm. Res, 3 (1), 47 – 51 (2012).
G. S. G. De Carvalho, P. A. Machado, D. T. S. de Paula, et al., Sci. World J., 10, 1723 – 1730 (2010).
G. K. Sharma, N. K. Sharma, and D. Pathak, Indian J. Chem, 52, 266 – 272 (2013).
J. M. Rademaker-Lakhai, D. V. D. Bongard, D. Pluim, et al., Clin. Cancer Res., 10, 3717 – 3727 (2004).
A. T. Baviskar, C. Madaan, R. Preet, et al, J. Med. Chem., 54(14), 5013 – 5030 (2011).
C. Santini, M. Pellei, V. Gandin, et al., Chem. Rev., 114(1), 815 – 862 (2014).
X. W. Liu, J. L. Y.-D. Chen, L. Li, and D.-S. Zhang, Inorg. Chim. Acta, 379, 1 – 6 (2011).
P. Skehan, R. Soreng, D. Scudiero, et al., J. Natl. Cancer Inst., 82(13), 1107 – 1112 (1990).
J. Wang, R. Mason, D. Van Derveer, et al., J. Org. Chem., 68(13), 5415 – 5418 (2003).
G. J. Lombardino and E. H. Wiseman, J. Med. Chem., 17(11), 1182 – 1188 (1974).
S. U. Rehman, T. Sarwar, M. A. Husain, et al., Arch. Biochem. Biophys., 576, 49 – 60 (2015).
J. D. McGhee and P. H. V. Hippel, J. Mol. Biol., 86 (2), 469 – 89 (1974).
S. Ramakrishnan and M. Palaniandavar, J. Chem. Sci., 117(2), 179 – 186 (2005).
S. Ramakrishnan, V. Rajendiran, M. Palaniandavar, et al., Inorg. Chem., 48(4), 1309 – 1322 (2009).
B. Selvakumar, V. Rajendiran, M. Palanaindavar, et al., J. Inorg. Biochem., 99(8), 1717 – 1732 (2006).
A. Terenzi, G. Barone, A. P. Piccionello, et al., Dalton Trans., 39(38), 9140 – 9145 (2010).
M. M. Heravi, K. Bakhtiari, H. A. Oskooie, and S. Taheri, J. Mol. Catal. A: Chem., 263, 279 – 281 (2007).
J. K. Barton and A. L. Raphael, J. Am. Chem. Soc., 106 (8), 2466 – 2468 (1984).
M. R. Eftink and C. A. Ghiron, Anal. Biochem., 114(2), 199 – 227 (1981).
S. Satyanarayana, J. C. Dabrowiak, and J. B. Chaires, Biochemistry, 32(10), 2573 – 2584 (1993).
S. Satyanarayana, J. C. Dabrowiak, and J. B. Chaires, Biochemistry, 31(39), 9319 – 9324 (1992).
J. M. Kelly, A. B. Tossi, D. J. McConnell, and C. Ohuigin, Nucl. Acids Res., 13(17), 6017 – 6034 (1985).
Y.-J. Liu, J. F. He, J.-H. Yao, et al., J. Coord. Chem., 62(4), 665 – 675 (2009).
X.-L. Hong, Z.-H. Liang, and M.-H. Zeng, J. Coord. Chem, 64(21), 3792 – 3807 (2011).
D. Canakci, I. Koyuncu, et al., J. Enzyme Inhib. Med. Chem., 34(1), 110 – 116 (2019).
A. Hussain, M. F. AlAjmi, et al., Sci. Rep., 9(1), 5237 (2019).
P. R. Inamdar, R. Chauhan, et al., Inorg. Chem. Commun., 67, 67 – 71 (2016).
L. Goodrich, H. Gerber, E. Marti, and D. F. Antczak, Vet. Clin. North Am. Equine Pract., 14, 607 – 629 (1998).
A. P. Theon, Vet. Clin. North Am. Equine Pract., 14, 659 – 671 (1998).
L. A. Fortier and M. A. MacHarg, J. Am. Vet. Med. Assoc., 205(8), 1183 – 1185 (1994).
S. Peterson, Vet. Rec., 141, 626 – 628 (1997).
Conflict of Interest
The authors declare that theyhave no conflictstoffinterest
Funding
The authors thank Zonguldak Bulent Ecevit University for sponsoring this project with a grant number 2020-72118496-02.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gerçek, Z., Yıldız, U., Ulukaya, E. et al. Synthesis, DNA Binding and Cytotoxic Activity of Newcopper(II) Complexes of Trisubstituted Imidazoles. Pharm Chem J 55, 1320–1328 (2022). https://doi.org/10.1007/s11094-022-02578-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-022-02578-2