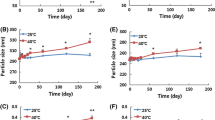
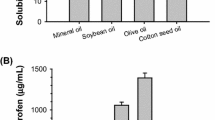
Due to poor aqueous solubility of paclitaxel, cremophor is one of the excipients used to improve solubility in Taxol while it is responsible for a number of adverse effects such as anaphylactic shock. This research was aimed to determine the solubility and stability of paclitaxel in the presence of intravenous injectable excipients. For this purpose, the solubility of paclitaxel was measured in PEG 400, ethanol, miglyol 812, octanoic acid and oleic acid using the shake flask method. Paclitaxel showed the highest solubility in PEG 400 because of possible hydrophobic interaction of the drug with polyethylene chains. The solubility of paclitaxel in ethanol was higher than its aqueous solubility, accentuating the importance of hydrogen bonding. The solubility of paclitaxel was slightly improved in octanoic acid, oleic acid and miglyol 812, presumably for less matching hydrophobic-hydrophilic balance of their molecules with paclitaxel. The stability of paclitaxel was examined in PEG 400, ethanol, and their binary mixture. Paclitaxel exhibited highest stability in the latter case. Probably, this is because of the matching polarity of PEG 400–ethanol mixture with paclitaxel. The effect of anhydrous citric acid on the stability of paclitaxel was also studied. Citric acid significantly improved the stability because it set pH of the prepared compositions in the range of 3 – 5, where paclitaxel exhibited the slowest rate of degradation. The prepared compositions were introduced in aqueous media in various concentrations to study precipitation due to dilution. Precipitation has been observed at all concentrations because paclitaxel is greatly insoluble in water and resists re-dissolving.
Similar content being viewed by others
References
M. E. Wall and M. C Wani, J. Ethnopharmacol., 51, 239 – 254 (1996).
M. S. Surapaneni, S. K. Das, and N. G. Das, ISRN Pharmacol., (2012).
A. O. Nornoo, D. W. Osborne, and D. L. Chow, Int. J. Pharm., 349, 108 – 116 (2008).
C. M. Spencer and D. Faulds, Drugs, 48, 794 – 847 (1994).
J. S. Kloover, M. A. den Bakker, H. Gelderblom, and J. P. van Meerbeeck, Brit. J. Cancer, 90, 304 – 305 (2004).
A. K. Singla, A. Garg, and D. Aggarwal, Int. J. Pharm., 235, 179 – 192 (2002).
J. A. Yared and K. Tkaczuk, Drug. Des. Devel. Ther., 6, 371 – 384 (2012).
J. T. Rubino and S. H. Yalkowsky, Pharm. Res., 4, 220 – 230 (1987).
M. El-Mahrab-Robert, V. Rosilio, M-A. Bolzinger, et al., Int. J. Pharm., 348, 89 – 94 (2008).
S. Reiner, G. Reineccius, and T. Peppard, J. Food. Sci., 75, 236 – 246 (2010).
C. Wohlfahrt and M. D. Lechner, Static Dielectric Constants of Pure Liquids and Binary Liquid Mixtures: Supplement to Volume IV / 17, Springer, Berlin (2015), pp. 216 – 217.
J. W. Millard, F. Alvarez-Nunez and S. H. Yalkowsky, Int. J. Pharm., 245, 153 – 166 (2002).
Canadian Institutes of Health Research. Oleic Acid. drugbank.ca/drugs/DB04224 (Accessed August 15, 2020).
Canadian Institutes of Health Research. Caprylic Acid. drugbank.ca/drugs/DB04519 (Accessed August 15, 2020).
Canadian Institutes of Health Research. PEG 400. drugbank.ca/drugs/DB03556 (Accessed August 15, 2020).
Canadian Institutes of Health Research. Ethanol. drugbank.ca/drugs/DB00898 (Accessed August 15, 2020).
Y. Qiu, Y. Chen, G. G. Zhang, et al., Developing Solid Oral Dosage Forms: Pharmaceutical Theory and Practice, Academic Press, New York (2009), pp. 5, 15, 94.
S. V. Balasubramanian, J. L. Alderfer, and R. M. Straubinger, J. Pharm. Sci., 83, 1470 – 1476 (1994).
D. Mastropaolo, A. Camerman, Y. Luo, et al., PNAS, 92, 6920 – 6924 (1995).
I. Nandi, M. Bateson, M. Bari, et al., AAPS Pharm. Sci. Tech., 4, 1 – 5 (2003).
Y. S. Thorat, I. D. Gonjari, and A. H. Hosmani, Int. J. Pharm. Sci. Res., 10(2), 2501 (2011).
V. R. Vemula, V. Lagishetty, and S. Lingala, Int. J. Pharm. Sci. Rev. Res., 5, 41 – 51 (2010).
Y. C. Lee, P. D. Zocharski, and B. Samas, Int. J. Pharm., 253, 111 – 119 (2003).
S. Kalepu and V. Nekkanti, Acta. Pharm. Sin. B., 5, 442 – 453 (2015).
International Conference on Harmonization. Q1A (R2): Stability Testing of New Drug Substances and Products (Revision 2); ich.org/products/guidelines/quality/article/quality-guidelines (Accessed August 15, 2020).
A. N. Martin, P. J. Sinko, and Y. Singh, Martin’s Physical Pharmacy and Pharmaceutical Sciences: Physical, Chemical and Piopharmaceutical Principles in the Pharmaceutical Sciences, Sixth edition, Lippincott Williams & Wilkins, Baltimore (2011), pp. 335 – 340.
T. Y. Ma, D. Hollander, P. Krugliak, and K. Katz, Gastroenterology, 98, 39 – 46 (1990).
E. Rytting, K. A. Lentz, X. Q. Chen, et al., Pharm. Res., 21, 237 – 244 (2004).
J. Tian and V. J. Stella, J. Pharm. Sci., 97, 3100 – 3108 (2008).
S. K. Dordunoo and H. M. Burt, Int. J. Pharm., 133, 191 – 201 (1996).
H. Montaseri, F. Jamali, J. A. Rogers, et al., Iran J. Pharm. Res., 1, 43 – 51 (2005).
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sadeghi-Oroumiyeh, A., Valizadeh, H. & Zakeri-Milani, P. Determination of Paclitaxel Solubility and Stability in the Presence of Injectable Excipients. Pharm Chem J 55, 983–987 (2021). https://doi.org/10.1007/s11094-021-02526-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-021-02526-6