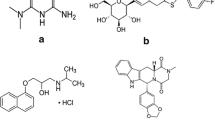
A sensitive, selective and rapid liquid chromatography–electrospray ionization–tandem mass spectrometry (LC-ESI-MS/MS) method has been developed for the estimation of mesalamine in human plasma using a derivatization technique. Aliquid-liquid extraction technique with ethyl acetate was used for the extraction of mesalamine and mesalamine-D3 internal standard (IS). Mesalamine and mesalamine-D3 were analyzed on Thermo BetaBasic C8 (100 mm × 4.6 mm, 5 μm) column using acetonitrile and 10 mM ammonium formate in 70 : 30 v/v ratio under isocratic elution mode. The MS detection and quantitation were performed under multiple reaction monitoring using positive ESI for mesalamine (m/z, 210.1 → 191.9) and mesalamine D3 (m/z, 213.2 → 195.1). Main advantages of the proposed method include higher sensitivity (5.00 ng/mL), superior extraction efficiency (≥79%), and small volume (200 μL) of sample for processing. The calibration curve was linear in the concentration range of 5.00 – 2957.17 ng/mL with a good correlation coefficient (r2 ≥ 0.9980) for mesalamine. The intra-batch (within day) and inter-batch (between days) precision (% CV) and accuracy (% nominal) values varied from 2.09 to 4.44% and from 96.02 to 103.23%, respectively. The method was validated as per the US FDA guidelines and the results were found accurate and precise.




Similar content being viewed by others
References
M. Ham and A. C. Moss, Expert Rev. Clin. Pharmacol., 5(2), 113 – 123 (2012).
M. D. Kappelman, K. R. Moore, J. K. Allen, et al., Dig. Dis. Sci., 58, 519 – 525 (2013).
L. Pastorelli, T. T. Pizarro, F. Cominelli, et al., Expert Opin. Emerging Drugs, 14(3), 505 – 521 (2009).
K. Gohil and B. Carramusa, Pharm. Ther., 39(8), 576 – 577 (2014).
E. V. Loftus, Gastroenterology, 126(6), 1504 – 1517 (2004).
U. Mahadevan, Clin. Colon. Rectal. Surg., 17(1), 7 – 19 (2004).
Drugbank (2019), Mesalazine. Accession Number: DB00244. https://www.drugbank.ca/drugs/DB00244 (accessed on November 10, 2019).
A. S. Kesselheim and J. J. Gagne, Drug Saf., 38(10), 849 – 853 (2015).
M. Štìpánková, R. Šelešovská, L. Janíková, et al., Chem. Pap., 71(8), 1419 – 1427 (2017).
T. N. Al-Sabha and N. N. Habeeb, Eur. Chem. Bull., 4(8), 384 – 388 (2015).
N. T. Dung, N. T. Anh, D. T. Oanh, et al., Vietnam J. Chem., 54(4), 509 – 514 (2016).
M. M. Morcoss, N. S. Abdelwahab, N. W. Ali, et al., Chem. Pharm. Bull., 64(9), 1268 – 1274 (2016).
L. Allen, J. Weinberger, and R. Weinberger, J. Chromatogr. A, 1053(1–2), 217 – 226 (2004).
J. Larsen, D. Staerk, C. Cornett, et al., J. Pharm. Biomed. Anal., 49(3), 839 – 842 (2009).
R. K. Trivedi, M. C. Patel and A. R. Kharkar, J. Chem., 8(1), 131 – 148 (2011).
M. Nobilis, Z. Vybiralova, K. Sladkova, et al., J. Chromatogr. A, 1119(1–2), 299 – 308 (2006).
K. S. Reddy, B. Ramachandran, and N. V. S. Naidu, Int. J. Sci. Eng. Res., 2(6), 52 – 56 (2014).
N. K. Sahoo, N. Sahu, P. S. Rao, et al., Pharm. Methods, 4(2), 56 – 61 (2013).
J. Qin, X. Di, X. Wang, et al., Biomed. Chromatogr., 29(2), 261 – 267 (2014).
G. Z. Gu, H. M. Xia, Z. Q. Pang, et al., J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 879(5–6), 449 – 456 (2011).
E. Pastorini, M. Locatelli, P. Simoni, et al., J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 872(1–2), 99 – 106 (2008).
K. Kanala, N. T. Hwisa, B. R. Chandu, et al., Brit. J. Pharm. Med. Res. 4(13), 1568 – 1590 (2014).
J. Banda, R. Lakshmanan, R. B. Katepalli, et al., J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 1008, 1 – 10 (2016).
H. Trufelli, P. Palma, G. Famiglini, et al., Mass Spectrom. Rev., 30(3), 491 – 509 (2011).
FDA Bioanalytical Method Validation Guidance for Industry, US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM), May 2001.
Acknowledgements
The authors acknowledge the Director, School of Pharmacy, Swami Ramanand Teerth Marathwada (SRTM) University (Nanded, India) for their support and encouragement to carry out this work. The authors would like to express their gratitude to the Chief Librarian of SRTM University (Nanded, India) for facilitating library to collect literature.
Financial Support
The authors received no financial support for the research and/or publication of this article.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pathan, M.M., Kshirsagar, A.D. Estimation of Mesalamine in Human Plasma Using Rapid and Sensitive LC-ESI-MS/MS Method. Pharm Chem J 55, 835–844 (2021). https://doi.org/10.1007/s11094-021-02504-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-021-02504-y