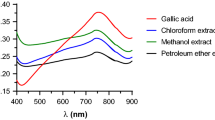
A dry extract of Ferula assa-foetida L. gum-resin was obtained. A formulation was developed on the basis of the extract and timogar preparation. Esters were qualitatively determined using the reaction with vanillin solution (1%) in conc. H2SO4; polysaccharides, with phenol in the presence of conc. H2SO4; coumarins, by TLC using umbelliferone standard. The quantitative content of timogar was determined using UV spectrophotometry; total phenolic compounds, the Folin–Ciocalteu method. The flavonoid content was also quantitatively determined. The formulation was standardized for description, solubility, mass loss on drying, heavy metals, authenticity (timogar, esters, polysaccharides, and coumarins), and quantitative content (timogar, total phenolic compounds, and flavonoids). The timogar content was 0.009 – 0.011%; total phenolic compounds, at least 75%. The flavonoid content was quantitatively determined using spectrophotometry and the reaction with an AlCl3 solution (10%) and should be at least 89% when recalculated using cynaroside as a standard. Experimental studies of acute and chronic toxicity showed that the proposed formulation was nontoxic and could be classified as hazard class 6 (relatively harmless). The immunostimulatory activity of the formulation exceeded by 1.3 times that of timogar alone.

Similar content being viewed by others
References
N. G. Saidova, G. Kh. Kodirova, and I. Dzh. Karomatov, Biol. Integr. Med., No. 9, 58 – 77 (2017).
A. A. Imanbaeva, K. N. Sarsenbaev, and M. S. Sagyndykova, Sib. Ekol. Zh., No. 6, 899 – 908 (2015).
S. Rakhimov, Biological and Morphological Characteristics of Ferula foetidissima Regelet Schmalh and Its Cultivation in Tajikistan [in Russian], Institute of Botany, AS Republic of Tajikistan, Dushanbe (2009).
T. M. Zubaidova, Dzh. N. Dzhamshedov, M. Khodzhimatov, et al., Vestn. Tajik. Nats. Univ., Ser. Estestv. Nauk, No. 1/2 (106), 202 – 212 (2013).
S. M. Bagheri, H. Mohammadsadeghi, M. H. Dashti-R, et al., Indian J. Nephrol. [serial online], 26, 419 – 422 (2016).
M. Mohammadhosseini, A. Venditti, S. D. Sarker, et al., Ind. Crops Prod., 129, 350 – 394 (2019).
G. Ouzek, I. A. Schepetkin, G. A. Utegenova, et al., J. Leukocyte Biol., 101, 1361 – 1371 (2017).
State Pharmacopoeia of the USSR, 7th Ed., Monograph 247, Gummi-resina asafoetida, Moscow (1937).
Pharmacopoeia of the People’s Republic of China, Beijing (2010), Vol. 1, 2.
M. A. Mamatkhanova, Kh. Akhmedova, L. D. Kotenko, et al., Uzb. Farm. Khabarnomasi, No. 4, 18 – 23 (2017).
L. D. Kotenko, M. A. Mamatkhanova, R. M. Khalilov, and A. U. Mamatkhanov, Khim. Rastit. Syr’ya, No. 4, 151 – 154 (2009).
L. D. Kotenko, R. M. Kahlilov, and A. U. Mamatkhanov, Khim. Rastit. Syr’ya, No. 1, 89 – 92 (2009).
A. A. Dehpour, M. A. Ebrahimzadeh, N. S. Fazel, and N. S. Mohammad, Grasas Aceites, 50(4), 405 – 412 (2009).
K. N. Mala, J. Thomas, D. S. Syam, et al., Evidence-Based Complementary Altern. Med., 2018, Art. ID 4813601; https://doi.org/10.1155/2018/4813601.
U. M. Khuseinov, Candidate Dissertation in Biological Sciences, Dushanbe (2019).
A. V. Kurkina, A. E. Savel’eva, and V. A. Kurkin, Khim.-farm. Zh., 55(2), 46 – 50 (2021); Pharm. Chem. J., 55(2), 165 – 169 (2021).
R. G. Farkhutdinov, N. V. Kudashkina, R. A. Zainullin, et al., Principles of Phytochemical Analysis [in Russian], RITs BashGU, Ufa (2016).
J. C. Sanchez-Rengel, J. Benavides, J. B. Heredia, et al., Anal. Methods, No. 5, 5990 – 5999 (2013).
A. N. Mironov (ed.), Handbook for Preclinical Drug Trials [in Russian], Grif i K, Moscow (2012), Part 1.
GOST 12.1.007–76. Labor Safety Standards System. Hazardous Substances. Classification and General Safety Requirements.
N. F. Izmerov, I. V. Sapotskii, and K. K. Sidorov, Acute Toxicity Parameters of Industrial Poisons [in Russian], Meditsina, Moscow (1977).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 55, No. 8, pp. 34 – 39, August, 2021.
Rights and permissions
About this article
Cite this article
Popova, O.A., Bunyatyan, N.D., Bobizoda, G.M. et al. Standardization of a Formulation of Timogar and Ferula Assa-Foetida Dry Resin Extract and its Biological Activity. Pharm Chem J 55, 787–792 (2021). https://doi.org/10.1007/s11094-021-02495-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-021-02495-w