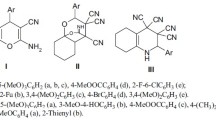
The antiproliferative activity of the previously synthesized 2-amino-4H-chromene-3-carbonitrile derivatives 4,7-disubstituted 3-cyanocoumarins and 2-aminochromeno[2,3-b]pyridine-3-carbonitriles was studied on 59 cell lines isolated from lung, colon, brain, ovary, kidney, prostate, and breast tumors and leukemia and human melanoma. The reference drugs were dacarbazine, hydroxycarbamide, and cisplatin. It was found that the activity of the test substances was directly related to the nature of the 4- and 7-substituents. 4-Aryl-2-oxo-2H-benzo [h]chromene-3-carbonitriles showed the most pronounced activity and could be of interest as parent substances for potential anticancer drugs.
Similar content being viewed by others
References
M. Costa, T. A. Dias, A. Brito, and F. Proenca, Eur. J. Med. Chem., 123, 487 – 507 (2016).
S. A. Patil, R. Patil, L. M. Pfeffer, and D. D. Miller, Future Med. Chem., 5(14), 1647 – 1660 (2013).
V. S. Moskvina and V. P. Khilya, Chem. Nat. Compd., 55(3), 401 – 427 (2019).
T. H. Afifi, F. F. Alblewi, A. M. El-Agrody, et al., Bioorg. Chem., 95, 103549 (2020).
N. D. Thanh, D. S. Hai, V. T. N. Bich, et al., Eur. J. Med. Chem., 167, 454 – 471(2019).
A. M. Fouda, A. M. S. Youssef, T. H. Afifi, et al., Med. Chem. Res., 28(5), 668 – 680 (2019).
T. H. Afifi, S. M. Riyadh, A. A. Deawaly, and A. Naqvi, Med. Chem. Res., 28(9), 1471 – 1487 (2019).
R. M. Okasha, M. Alsehli, S. Ihmaid, et al., Bioorg. Chem., 92, 103262 (2019).
I. N. Bardasov, A. Yu. Alekseeva, O. V. Ershov, and M. A. Mar’yasov, Khim.-farm. Zh., 54(5), 30 – 32 (2020); Pharm. Chem. J., 54(5), 459 – 461 (2020).
M. N. Semenova, D. V. Tsyganov, O. R. Malyshev, et al., Bioorg. Med. Chem. Lett., 24(16), 3914 – 3918 (2014).
I. N. Bardasov, A. U. Alekseeva, O. V Ershov, et al., Tetrahedron Lett., 56(44), 6145 – 6148 (2015).
D. R. Anderson, S. Hegde, E. Reinhard, et al., Bioorg. Med. Chem. Lett., 15(6), 1587 – 1590 (2005).
L. Zhang, X. Zhou, P. Li, et al., RSC Adv., 7(62), 39216 – 39220 (2017).
I. N. Bardasov, A. U. Alekseeva, D. L. Mihailov, et al., Tetrahedron Lett., 56(14), 1830 – 1832 (2015).
I. N. Bardasov, A. Y. Alekseeva, N. L. Malyshkina, et al., Russ. J. Org. Chem., 52(6), 830 – 833 (2016).
E. A. Melekhin, I. N. Bardasov, O. V. Ershov, et al., Russ. J. Org. Chem., 42(4), 622 – 623 (2006).
A. Monks and D. Scudiero, J. Natl. Cancer Inst., 83(11), 757 – 766 (1991).
https: //dtp.cancer.gov/discovery development/nci-60/cell list.htm.
GPM. 1.1.0014.15, State Pharmacopoeia of the Russian Federation, XIVth Ed., Vol. 1 (2018), pp. 319 – 369; http: //femb.ru/femb/pharmacopea.ph.
F. Alblewi, R. Okasha, A. Eskandrani, et al., Molecules, 24(6), 1060 (2019).
H. E. A. Ahmed, M. A. A. El-Nassag, A. H. Hassan, et al., J. Enzyme Inhib. Med. Chem., 33(1), 1074 – 1088 (2018).
R. Okasha, F. Alblewi, T. Afifi, et al., Molecules, 22(3), 479 (2017).
Acknowledgments
The work was performed in the framework of a State Task for the Ministry of Education and Science of Russia, Project No. 0849-2020-0003.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 55, No. 7, pp. 14 – 17, July, 2021.
Rights and permissions
About this article
Cite this article
Bardasov, I.N., Alekseeva, A.Y., Ershov, O.V. et al. Antiproliferative Activity of 3-Cyanocoumarins and 2-Aminochromeno[2,3-b ]Pyridine-3-Carbonitriles, Derivatives of 2-Amino-4H-Chromene-3-Carbonitrile. Pharm Chem J 55, 644–647 (2021). https://doi.org/10.1007/s11094-021-02473-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-021-02473-2