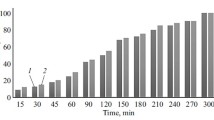
Results from studies of the stability of the antiallergy gel for external application Fludigel on long-term storage in natural conditions are presented. The gel was stored at +25 ± 10°C in 25-g plastic tubes. Three batches of product were assessed in terms of the main quality indicators: description, identity, pH, colloid and thermal stability, assay, and microbial contamination. In addition, stability was studied by microscopic examination using scanning electron microscopy. These studies demonstrated stability in the quality measures of three batches of gel and a shelf life of two years was established

Similar content being viewed by others
References
State Pharmacopeia of Russian Federation [in Russian], N. V. Yurgel′ (ed.), Meditsina (2008), XII edition, (Part 1).
State Pharmacopeia of Russian Federation [in Russian], N. V. Yurgel′ (ed.), Meditsina (2008), XIII edition, Part 1, General Pharmacopeia Monograph OFS1.009.5 “Shelf lives of medicines.”
Guidelines for Expert Assessment of Medicines. Studies of Stability and Determination of the Shelf Life of Medicines [in Russian], Poligraf-Plyus, Meditsina, Moscow (2014), Vol. III.
A. V. Filatova, A. S. Turaev, and D. T. Dzhurabaev, Khim. Khim. Tekhnol., No. 3, 52 – 55 (2019).
A. V. Filatova, D. T. Dzhurabaev, and A. S. Turaev, Uzbek. Khim. Zh., No. 4, 60 – 63 (2015).
M. M. Astrakhanova, Farmatsiya, No. 6, 55 – 59 (2007).
V. L. Bagirova, E. L. Kovaleva, and N. K. Sadchikova, Khim.-Farm. Zh., No. 11, 46 – 47 (2000).
N. P. Maksyutina, F. B. Kagan, L. A. Kirichenko, and F. A. Mitchenko, Methods for Drug Analysis [in Russian], Zdorov′e, Kiev (2005).
State Standard GOST 33756-2016. Plastic Consumer Packaging. General Technical Conditions.
State Standard GOST 29188.0–91. Perfumery-Cosmetic Products. Rules for Acceptance, Sample Selection, and Organoleptic Study Tests.
A. Kh. Laipanov, Farmatsiya, 40(1), 28 – 31 (1991).
State Standard GOST 29188.2–91. Cosmetic Products. Methods for Determining pH. Replacement for Industrial standard OST 18-304–76, para. 3.10; state standard GOST 7983–82, para. 3.8; introduced January 1, 1993. Standards Press, Moscow (1992), p. 3. UDC 665.58.001.4.006.354. Group R 19.
State standard GOST 29188.3–91. Cosmetic Products. Methods for determining the stability of emulsions.
Industrial standard OFS. 1.2.4.0002.15, Microbial contamination. Replacement for State Pharmacopeia, XI edition, Part 2, pp. 193, Amendments Nos. 2 and 3; Industrial standard OFS 42-0016-04, F XII, ch. 1, OFS 42-0067-07.
V. A. Kodintseva, Z. D. Khadzhieva, and V. F. Dzyuba, Proceedings of All-Russian Congress of Pharmaceutical Workers [in Russian], Moscow (2014), pp. 128 – 130.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 54, No. 11, pp. 53 – 56, November, 2020.
Rights and permissions
About this article
Cite this article
Filatova, A.V., Turaev, A.S., Dzhurabaev, D.T. et al. Stability of the Combined Antiallergic Gel Fludigel. Pharm Chem J 54, 1169–1172 (2021). https://doi.org/10.1007/s11094-021-02337-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-021-02337-9