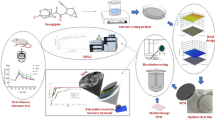
The discovery and development of drugs that can compete in effectiveness with direct anticoagulants is a critical problem. Fucoidan is a complex branched heteropolysaccharide with anticoagulant and antithrombic activity. Fucoidan drug substance is hygroscopic and possesses poor technological properties. This study was aimed at development of the optimum composition and technology of fucoidan tablets and their biopharmaceutical evaluation. The generalized Harrington desirability function and dispersion and regression analyses were used for optimization of the fucoidan tablet formulation. The contents of crospovidone and lactose were the most significant factors affecting the disintegration and compressibility of model tablets. The lactose content had the greatest impact on the Carr index and Hausner ratio. The in vitro dissolution curves of the tablets were compared (at pH 1.2, 5.7, and 6.8). The in vitro release of fucoidan from the developed tablets obeyed a first-order kinetic equation.


Similar content being viewed by others
References
N. A. Ushakova, G. E. Morozevich, N. E. Ustyuzhanina, et al., Biomed. Khim., 54(5), 597 – 606 (2008).
A. G. Odinets and L. V. Tatarinova, Lech. Delo, 49(3), 40 – 44 (2016).
J. H. Fitton, D. N. Stringer, A. Y. Park, and S. S. Karpiniec, Mar. Drugs, 17, 571 (2019).
E. I. Oduah, R. J. Linhardt, and S. T. Sharfstein, Pharmaceuticals, 9(3), 38 (2016).
S. K. Zyryanov and Yu. B. Belousov, Aterotromboz, No. 1, 38 – 43 (2013).
B. L. Limone, A. V. Hernandez, D. Michalak, et al., Thromb. Res., 132(4), 420 – 426 (2013).
M. V. Khruslov, Flebologiya, 9(3), 41 – 46 (2015).
S. V. Konstantinides, A. Torbicki, G. Agnelli, et al., Eur. Heart J., 35(43), 3033 – 3069 (2014).
N. N. Besednova and T. N. Zvyagintseva (eds.), Fucoidans, Sulfated Polysaccharides from Brown Algae. Structure, Enzymatic Transformation and Biological Properties [in Russian], Vladivostok (2014).
X. Zhao, F. Guo, J. Hu, et al., Thromb. Res., 144, 46 – 52 (2016).
O. N. Pozharitskaya, A. N. Shikov, N. M. Faustova, et al., Mar. Drugs, 16(4), 132 (2018).
M. R. Irhimeh, J. H. Fitton, and R. M. Lowenthal, Blood Coagul. Fibrinolysis, 20(7), 607 – 610 (2009).
E. D. Obluchinskaya, M. N. Makarova, O. N. Pozharitskaya, and A. N. Shikov, Khim.-farm. Zh., 49(3), 35 – 38 (2015); Pharm. Chem. J., 49(3), 183 – 186 (2015).
S. N. Bykovskii (ed.), Pharmaceutical Development: Concept and Practical Recommendations. Scientific-Practical Guideline for the Pharmaceutical Branch [in Russian], Pero, Moscow (2015).
V. M. Kosman, E. D. Obluchinskaya, O. N. Pozharitskaya, et al., Farmatsiya, 66(6), 20 – 24 (2017).
H. K. Raslan and H. Maswadeh, Indian J. Pharm. Sci., 68, 308 – 312 (2006).
A. V. Pichkalev, Issled. Naukograda, 1 (2012).
N. V. Slovesnova, A. Yu. Petrov, S. A. Glavatskikh, et al., Razrab. Regist. Lek. Sredstv, 23(2), 32 – 37 (2018).
D. V. Yudina, E. V. Blynskaya, K. V. Alekseev, et al., Farmatsiya, 67(3), 35 – 40 (2018).
S. L. Akhnazarova and L. S. Gordeev (eds.), Use of the Harrington Desirability Function to Optimize Chemical Technology. Study-Methodology Guide [in Russian], RKhTU im. D. S. Mendeleeva, Moscow (2003).
Methodical Instructions for Drug Bioequivalence Assessment [in Russian], MZSR RF, Moscow (2008).
E. V. Stoyanov and R. Vollmer, Prom. Obozrenie, 4(15), 48 – 49 (2009).
F. T. Kholtoev, N. S. Faizullaeva, M. U. Usubbaev, and Kh. M. Khakimov, Khim.-farm. Zh., 37(6), 42 – 45 (2003); Pharm. Chem. J., 37(6), 321 – 324 (2003).
E. D. Obluchinskaya, Khim.-farm. Zh., 43(6), 22 – 26 (2009); Pharm. Chem. J., 43(6), ID 328 (2009).
K. Kadena, M. Tomori, M. Iha, and T. Nagamine, Mar. Drugs, 16(8), E254 (2018).
Y. Tokita, M. Hirayama, K. Nakajima, et al., J. Nutr. Sci. Vitaminol., 63(6), 419 – 421 (2017).
T. I. Imbs, T. N. Zvyagintseva, and S. P. Ermakova, Int. J. Biol. Macromol., 142, 778 – 781 (2020).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 54, No. 5, pp. 38 – 42, May, 2020.
Rights and permissions
About this article
Cite this article
Obluchinskaya, E.D., Pozharitskaya, O.N., Flisyuk, E.V. et al. Optimization of the Composition and Production Technology of Fucoidan Tablets and their Biopharmaceutical Evaluation. Pharm Chem J 54, 509–513 (2020). https://doi.org/10.1007/s11094-020-02237-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-020-02237-4