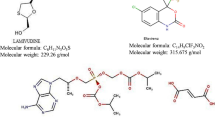
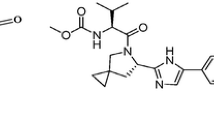
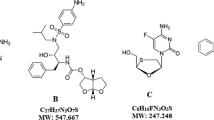
A new simple, precise and robust isocratic reverse-phase high performance liquid chromatography (RP-HPLC) method was developed and validated for simultaneous determination of lamivudine, tenofovir disoproxil fumarate (TDF), and doravirine in bulk and pharmaceutical dosage form. The validation included specificity, linearity, system suitability, precision, robustness, LOD and LOQ characteristics. The chromatographic separation was achieved on C18X bridge phenyl column (150 × 4.6 mm, 3 μm particle size) eluted with acetonitrile and hexane-1-sulfonic acid (pH 2.5; 50:50, v/v) at a flow rate of 0.8 mL/min and monitored at 243 nm over a run time of 12 min. The retention times of lamivudine, TDF, and doravirine were found to be 2.45, 7.3, and 8.79 min. respectively. The method was linear in the range of 5 – 100 μg/mL (r2 = 0.999) for lamivudine and TDF and in the range of 1.75 – 35 μg/mL (r2 = 0.999) for doravirine. The percentage recoveries of three drugs were within the acceptable limits (98 – 102%). The method was found to be precise as confirmed by % RSD < 0.6. Forced degradation study was conducted as per ICH guidelines, and the three drugs showed degradation within 21.4 – 33.8% under acidic, basic, oxidative, photolysis, and hydrolysis conditions. The proposed RP-HPLC method can be used for the quantification of lamivudine, TDF, and doravirine in API and tablets without any interference from excipients.









Similar content being viewed by others
References
K. Nahiro, J. Antimicrob. Chemother., 51, 1085 – 1089 (2003)
E. G Joel and D. Stanley, Clin. Infect. Dis., 37, 944 – 950 (2003).
B. Maria and H. Maria, Expert Rev. Gastroenterol. Hepatol., 6, 413 – 421 (2012)
C. Nicolas Sluis and T. Gilda, Virus Res., 134, 147 – 156 (2008).
R. Surendra, C. Tejasri, K. Dharmendra, et al., J. Pharm, Biomed. A: Lett., 4, 70 – 73 (2016).
J. Guo, F. Meng, L. Li, et al., Biol. Pharm. Bull., 34, 877 – 882 (2011).
S. O. Choi, N. Rezk, J. S. Kim, and A. D. Kashuba, J. Chromatogr. Sci., 48, 219 – 223 (2010).
M. Alebouyeh and H. Amini, J. Chromatogr. B: Analyt. Technol. Biomed. Life Sci., 975, 40 – 44 (2015).
G. Bahrami, S. Mirzaeei, A. Kiani, and B. Mohammadi, J. Chromatogr. B: Analyt. Technol. Biomed. Life Sci., 823(2), 213 – 7 (2005).
V. S. Akhilesh, K. N. Lila, and R. P. Nihar, J. Pharm. Anal., 1(4), 251–257 (2011).
W. Kromdijk, S. A. Pereira, and H. Rosing, J. Chromatogr. B: Analyt. Technol. Biomed. Life Sci., 919 – 920, 43 – 51 (2013).
C. Waitt, S. Diliiy Penchala, and A. Olagunju, J. Chromatogr. B: Analyt. Technol. Biomed. Life Sci., 1060, 300 – 307 (2017).
D. S. Bhavsar, B. N. Patel, and C. N. Patel, Pharm. Methods, 3(2), 73 – 8 (2012).
R. K. Valluru, B. B. Reddy, S. K. Sumanth, et al., J. Chromatogr. B: Analyt. Technol. Biomed. Life Sci., 931, 117 – 126 (2013).
N. Mallikarjuna Rao and D. Gowri Sankar, Futur. J. Pharm. Sci., 1(2), 73 – 77 (2015).
Dhara S. Bhavsar, B. N. Patel, and C. N. Patel, Pharm. Methods, 3(2), 73 – 78 (2012).
ICH Harmonized Tripartite Guideline, Validation of Analytical Procedures: Text and Methodology, Q2 (R1): International Conference on Harmonization, IFPMA, Geneva, Switzerland (2005).
E. Tamizi and A. Jouyban, Eur. J. Pharm. Biopharm., 98, 26 – 46 (2016).
M. Blessy, D. Ruchi, N. Prajesh, et al., J. Pharm. Anal., 4, 159 – 165 (2014).
Acknowledgements
The authors are thankful to Maharajah’s College of Pharmacy (Vizianagaram, India) and GITAM Institute of Pharmacy (Visakhapatnam, India) for supporting and providing necessary facilities for the research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gollu, G., Gummadi, S. Simultaneous Quantification of Lamivudine, Tenofovir Disoproxil Fumarate and Doravirine in Pharmaceutical Dosage Form by Liquid Chromatography with Diode Array Detection. Pharm Chem J 54, 526–535 (2020). https://doi.org/10.1007/s11094-020-02232-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-020-02232-9