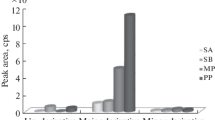
Anew method for quantitative determination of the active ingredient in phenyl salicylate drug substance using gas chromatography with flame-ionization detection (GC-FID) was developed and validated. The experiments used an HP-5 quartz capillary column with a grafted stationary phase of diphenyl- (5%) and dimethylpolysiloxane (95%) (Agilent Technologies, USA). The conditions for the chromatographic separation and the chromatography system suitability parameters were determined. The phenyl salicylate content in the drug substance was determined by the internal standard method. The method was validated for specificity, linearity, precision, and stability (robustness) and was found to meet the main acceptance criteria.




Similar content being viewed by others
References
State Pharmacopoeia of the USSR, Xth Ed., Meditsina, Moscow (1968), pp. 530 – 531.
State Pharmacopoeia of the RF, XIIIth Ed., Moscow (2015), Vol. 1, pp. 490 – 495.
State Pharmacopoeia of the RF, XIIIth Ed., Moscow (2015), Vol. 1, pp. 447 – 474.
State Pharmacopoeia of the RF, XIIIth Ed., Moscow (2015), Vol. 1, pp. 121 – 129.
J. Krzek, J. S. Czekaj, and W. Rzeszutko, Acta Pol. Pharm., 60(5), 343 – 349 (2003).
K. Mori, K. Terajima, Y. Nakamura, et al., J. Tokyo-toritsu Eisei Kenkyusho Kenkyu Nenpo, 53, 65 – 67 (2003).
State Pharmacopoeia of the RF, XIIIth Ed., Moscow (2015), Vol. 1, pp. 235 – 264.
State Pharmacopoeia of the RF, XIIIth Ed., Moscow (2015), Vol. 1, pp. 222 – 234.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 54, No. 1, pp. 43 – 48, January, 2020.
Rights and permissions
About this article
Cite this article
Kupriyanova, O.V., Milyukov, V.A., Shevyrin, V.A. et al. Development and Validation of a Gas Chromatographic Method for Quantitative Determination of the Active Ingredient in Phenyl Salicylate Drug Substance. Pharm Chem J 54, 73–78 (2020). https://doi.org/10.1007/s11094-020-02159-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-020-02159-1