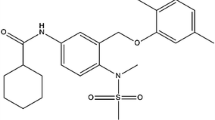
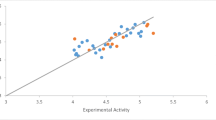
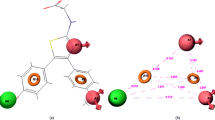
Interleukin-6 (IL-6) is the new molecular target to treat colorectal cancer (CRC) for investigation of potential lead molecules. The main objective of this study was to develop the three-dimensional quantitative structure – activity relationship (3D-QSAR) and pharmacophore model using data obtained for HT-29 cells. In this study, common pharmacophore model and atom-based 3D-QSAR were generated using newly reported 1,3,4-thiadiazole scaffolds. The dependable common pharmacophore hypothesis (AARR53) and statistically significant 3D-QSAR models were generated, where the highest correlation coefficient (r2 = 0.94) for the best-fit linear relation y = 0.96x + 0.07 was obtained by partial least squares (PLS) analysis. Again, VR series molecules displayed ligand-protein complex stability during molecular dynamics (MD) simulations. The PHASE predicted activity data and LogGI50 values demonstrated most significant atomic positions in the backbone structure of ligands in order to establish effectiveness with respect to CRC activity. Furthermore, molecular docking and dynamics simulations were carried out between top rank lead and IL-6 target which provided and defined the better binding conformational and complex stability into the active pocket of target throughout the MD simulation. These data were further confirmed through in vivo dimethylhydrazine (DMH) induced CRC rat model where it was found that both VR24 and VR27 reduced the concentration of IL-6 at the CRC site as compared to DMH control. This observation again supported computational simulation hypothesis and revealed that both compounds had good IL-6 reduction property at CRC site. The final outcome from this design strategy indicated that the pharmacophore models and 3D-QSAR hypothesis might be a path of milestone in the area of medicinal chemistry for further design of new and potent IL-6 inhibitors.







Similar content being viewed by others
References
T. Hirano, K. Yasukawa, H. Harada, et al., Nature, 324, 73 – 76 (1986).
K. Ishihara and T. Hirano, Cytokine Growth Factor Rev., 13, 357 – 368 (2002).
M. Nian, P. Lee, N. Khaper, et al., Circ. Res.,94, 1543 – 1553 (2004).
R. Kurzrock, Clin. Cancer Res., 3, 2581 – 2584(1997).
M. Trikha, R. Corringham, B. Klein, et al., Clin. Cancer Res., 9, 4653 – 4665 (2003).
R. A. Burger, E. A. Grosen, G. R. Ioli, et al., J. Interferon Cytokine Res., 15, 255 – 260 (1995).
C. Wu, S. Wang, M. Chao, et al., Am. J. Gastroenterol, 91, 1417 – 1422 (1996).
C. Lu, C. Sheehan, and J. W. Rak, Clin. Cancer Res., 2, 1417 – 1425 (1996).
M. Oka, N. Iizuka, K. Yamamoto, et al., J. Interferon Cytokine Res., 16, 1001 – 1006 (1996).
M. Kawano, T. Hirano, T. Matsuda, et al., Nature, 332, 83 – 85 (1988).
S. Miki, M. Iwano, Y. Miki, et al., FEBS Lett., 250, 607 – 610 (1989).
F. Riedel, I. Zaiss, D. Herzog, et al., Anticancer Res., 25, 2761 – 2765 (2005).
R. Salgado, S. Junius, I. Benoy, et al., Int. J. Cancer,103, 642 – 646 (2003).
D. J. George, S. Halabi, T. F. Shepard, et al., Clin. Cancer Res., 11,1815 – 1820 (2005).
T. Kinoshita, H. Ito, and C. Miki, Cancer, 85, 2526 – 2531(1999).
Y. C. Chung and Y. F. Chang, J. Surg. Oncol., 83, 222 – 226 (2003).
T. Kishimoto, S. Akira, and M. Narazaki, Blood, 86, 1243 – 1254 (1995).
T. Taga and T. Kishimoto, Annu. Rev. Immunol.,15, 797 – 819(1997).
P. C. Heinrich, I. Behrmann, H. Serge, Biochem. J.,374, 1 – 20 (2003).
T. Hirano, K. Ishihara, and M. Hibi, Oncogene, 19, 2548 – 2556 (2000).
S. H. Jee, C. Y. Chu, H. C. Chiu, et al., J. Invest. Dermatol., 123, 1169 – 1175 (2004
C. M. Leu, F. H. Wong, C. Chang, et al., Oncogene, 22, 7809 – 7818 (2003).
M. Köbel, D. Budianto, and W. D. Schmitt, Oncology, 68, 33 – 39 (2005).
H. Lahm, D. Petral-Malec, A. Yilmaz-Ceyhan, et al., Eur. J. Cancer, 28, 1894 – 1899 (1992).
R. Vinit, R. Amit, S. K. Ashok, et al., Anti-Cancer Agents Med. Chem. BSP-ACAMC-2016 – 1059 (2016).
E. De Boer, W. De Jong, and P. Steerenberg, Cancer Immunol. Immunother., 34, 306 – 312 (1992).
E. Krieger and G. Vriend, J. Comput. Chem., 36, 996 – 1007 (2015).
R. B. Best, N. V. Buchete, and G. Hummer. Biophys. J., 95, L07 – L09 (2008).
M. Hirose, H. Yada, K. Hakoi, et al., Carcinogenesis,14, 2359 – 2364 (1993).
S. Landi, V. Moreno, L. Gioia-Patricola, et al., Cancer Res., 63, 3560 – 3566 (2003).
J. Małgorzata, M. Joanna, N. Andrzej, et al., Folia Histochem. Cytobiol.,49, 36 – 44 (2011).
R. Vinit, R. Amit, and S. Sudipta, Anti-Cancer Agents Med. Chem.,16, 1 (2016)
R. F. Asbury, A., Kramar, and D. G. Haller, Am. J. Clin. Oncol., ;10, 380 – 382 (1987).
Acknowledgments
The authors would like to express their gratitude to the Babasaheb Bhimrao Ambedkar University (Lucknow) for providing the research facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raj, V., Aboumanei, M.H., Rai, A. et al. Pharmacophore and 3d-Qsar Modeling of new 1,3,4-Thiadiazole Derivatives: Specificity to Colorectal Cancer. Pharm Chem J 54, 12–25 (2020). https://doi.org/10.1007/s11094-020-02149-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-020-02149-3