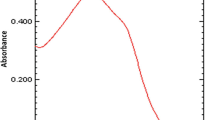
The purpose of this work was to validate the method of UV absorption spectrophotometry for the determination of thiamazole in transdermal patches during their pharmaceutical development. According to the pharmacopoeial requirements of the Republic of Kazakhstan, dissolution test was used to assess the quality of drug product. Measuring the optical density of thiamazole solutions at a wavelength of 252 nm in the range of concentrations from 0.005 to 0.04 mg/mL showed that the optimal test solution concentration is 0.005 mg/mL. The proposed method was validated through the standard and drug recovery method for linearity, range, accuracy and precision. Optimized and validated, it demonstrated high specificity and accuracy for transdermal patch with thiamazole. The obtained results proved that the method could be used for routine analysis as well as for pharmaceutical development and quality control for thiamazole patches.





Similar content being viewed by others
References
A. Fumarola, A. Di Fiore, M. Dainelli, et al., Exp. Clin. Endocrinol. Diabetes, 118(10), 678 – 684 (2010).
B. L. Furman, in: Reference Module in Biomedical Sciences (2017).
L. Gales and J. K. Aronson, Side Effects Drugs Ann., 35, 747 – 761 (2014).
X. Wu, H. Liu, X. Zhu, et al., Environ. Toxicol. Pharmacol., 36(3), 1109 – 1112 (2013).
K. E. Hill, M. A. Gieseg, D. Kingsbury, et al., J. Vet. Med., 25(6), 1357 – 1365 (2011).
D. Sanjay and A. Malgope, Int. J. Pharm. Pharm. Sci., 2(1), 137 – 143 (2010).
K. Hamang, M. Subrata, and K. Asif, Int. Res. J. Pharm., 3(11), 109 – 113 (2012).
Guidelines on Quality of Transdermal Patches, Committee for Medicinal Products for Human Use, CHMP (2014).
S. C. Gad, Pharmaceutical Manufacturing Handbook: Regulations and Quality (2013), Vol. 6, 831–898.
S. O. Losenkova and E. F. Stepanova, Sci. Lists Belgorod State Univ., 16(111), 15 (2011).
United States Pharmacopeia & National Formulary, United States Pharmacopeial Convention. Inc. (2016), Vol. 39, p. 3577
F. Jalali, L. Miri, and M. Roushani, Afric. J. Pharm. Pharmacol., 7(6), 269 – 274 (2013).
Z. Sheng, H. Han, G. Yang, Luminescence, 26, 196 – 201 (2011).
L. Özcan and Y. Şahin, Sensors Actuators B: Chemical., 127(2), 362 – 369 (2007).
T. A. Saleh, M. M. Al-Shalalfeh, and A. A. Al-Saadi, Sci. Rep., 6, 32185 (2016).
L. Farzampour and M. Amjadi, J. Lumin., 155, 226 – 230 (2014).
H. Ebrahimsadeh, A. A. Asgharinezhad, L. Adnasab, and N. Shekari, J. Separ. Sci., 35(16), 2040 – 2047 (2012).
G. Chvatko, V. M. Darras, and E. Bald, Food Add. Contam. Part A: Chem. Anal. Control Exp. Risk Assess, 31(6), 1009 – 1016 (2014).
European Pharmacopoeia-2016, Strasbourg, Council of Europe, Vol. 2, 1084 (2016).
B. Vaghela, R. Kayastha, N. Bhatt, et al., J. Appl. Pharm. Sci., 1(3), 50 – 56 (2011).
G. P. Martin and V. A. Gray, J. Valid. Technol., 1, 8 – 14 (2011).
The Japanese Pharmacopoeia, 17 Ed., Ministry of Health, Labor and Welfare (2016), p. 2629.
C. Carella, G. Mazziotti, F., Sorvillo, et al., Thyroid, 16(3), 295 – 302. (2006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Datkhayev, U.M., Sakipova, Z.B., Ustenova, G.O. et al. Validation of Spectrophotometric Method for Determination of Thiamazole in Liquids by Dissolution Test for the Transdermal Form. Pharm Chem J 53, 572–576 (2019). https://doi.org/10.1007/s11094-019-02039-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-019-02039-3