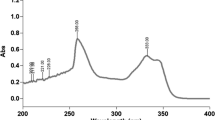
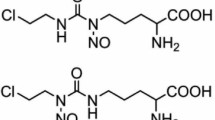
A spectrophotometric assay for ormustine active ingredient for a liposomal dosage form with antitumor activity was selected and validated for specificity, linearity, accuracy, precision, and intermediate (intralaboratory) precision in order to ensure accurate and precise results. The obtained statistical characteristics were shown to satisfy acceptance criteria for the validation parameters given in domestic regulatory documentation. Ormustine was determined at 396 ± 2 nm because excipients in the dosage form did not absorb in this spectral region. The correlation coefficient for the linearity was >0.997. The relative error of the mean result was <1% for the accuracy. The confidence interval included 100%. The coefficient of variation for the precision and intermediate precision determinations was <1%. The studied method could be used in the range 80 – 120% of the nominal ormustine content in the liposomal dosage form.



Similar content being viewed by others
References
A. V. Lantsova, E. V. Sanarova, and N. A. Oborotova, Biofarm. Zh., 6(5), 38 – 51 (2014).
Market for Oncological Drugs: Growth Areas and Development Prospects; http://clinvest.ru/news/item/rynokonkologicheskih-preparatov-tochki-rosta-i-perspektivy-razvitiya
IMS Institute, Innovation in Cancer Care and Implications for Health Systems. Global Oncology Trend Report (2014).
RU Pat. 256,729; Byull. Izobret., No. 33 (2015).
N. S. Saprykina, L. M. Borisova, M. P. Kiseleva, et al., Ross. Bioterapevticheskii Zh., 16(4), 55 – 60 (2017).
A. V. Lantsova, A. P. Polozkova, N. M. Peretolchina, et al., Ross. Bioterapevticheskii Zh., 3(4), 19 – 23 (2004).
L. L. Nikolaeva, A. V. Lantsova, E. V. Sanarova, et al., Nanotechnol. Russ., 11(5 – 6), 371 – 376 (2016).
S. I. Guseva, M. V. Karlina, O. N. Pozharitskaya, et al., Khimfarm. Zh., 44(1), 46 – 49 (2010); Pharm. Chem. J., 44(1), 43 – 46 (2010).
V. V. Beregovykh, Validation of Analytical Methods for Drug Production [in Russian], Litterra, Moscow (2008).
M. S. Goizman, N. L. Shimanovskii, D. L. Shobolov, et al., Khim.-farm. Zh., 52(6), 53 – 60 (2018); Pharm. Chem. J., 52(6), 562 – 568 (2018).
GPM 1.1.0013.15. Statistical processing of chemical experimental results; http://pharmacopoeia.ru/ofs-1-1-0013-15-statisticheskaya-obrabotka-rezultatov-eksperimenta/
GPM 1.1.0012.15. Validation of analytical methods; http://pharmacopoeia.ru/ofs-1-1-0012-15-validatsiya-analiticheskihmetodik/
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 52, No. 9, pp. 52 – 55, September, 2018.
Rights and permissions
About this article
Cite this article
Bunyatyan, N.D., Oborotova, N.A., Nikolaeva, L.L. et al. Validation of an Assay Procedure for Ormustine in a Liposomal Dosage Form. Pharm Chem J 52, 799–802 (2018). https://doi.org/10.1007/s11094-018-1903-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-018-1903-5