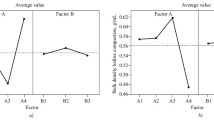
The influence of the isomalt mass fraction in a mixture with glucose on the flowability, density, and elastic-plastic properties of the tableting mass and the quality of the obtained 1-g tablets (14 mm diameter) was studied. The flowability of the mixture decreased by 0.6 ± 0.06 g/c if the isomalt ST-PF content was increased by 10%. The curve for the bulk density had a distinct maximum near an isomalt–glucose ratio of 50:50. The tablet strength increased linearly by 55 ± 7 and 51 ± 7 N; the density, by 0.0036 ± 0.0002 and 0.076 ± 0.003 g/cm3; and the disintegration time, by 1.2 ± 0.2 and 1.7 ± 02 min if the isomalt ST-PF concentration in the mixture was increased to 35% and the compaction pressure to 350 N/cm2, respectively.





Similar content being viewed by others
References
N. N. Zhuikova, O. S. Sablina, E. A. Shtokareva, and A. S. Gavrilov, Khim.-farm. Zh., 43(8), 50 – 52 (2009); Pharm. Chem. J., 43(8), Article: 447 (2009).
K. Day, K. Xiao, et al., US Pat. 8,383,632, Feb. 26, 2013.
E. V. Al’shev, RU Pat. 2,426,454, Dec. 27, 2010.
C.-Y. Wu, B. C. Hancock, A. Mills, et al., Powder Technol., 181, 121 – 129 (2008).
G. K. Bolhuis, J. J. Engelhart, and A. C. Eissens, Eur. J. Pharm. Biopharm., 72(3), 621 – 625 (2009).
G. K. Bolhuis E. G. Rexwinkel, and K. Zuurman, Drug Dev. Ind. Pharm., 35(6), 671 – 677 (2009).
W. L. Chen, D. W. Guo, Y. Y. Shen, et al., Indian J. Pharm. Sci., 74(6), 527 – 534 (2012).
M. Smuda, Angew. Chem., Int. Ed., 52(18), 4887 – 4891 (2013).
J. Muzíková and V. Pavlasová, Cesko-Slov. Farm., 60(1), 11 – 16 (2011).
M. B. Val’ter, O. L. Tyutenkov, and N. A. Fillipin, Staged Control in Tablet Manufacturing [in Russian], Meditsina, Moscow (1982), pp. 19 – 49.
R. W. Heckel, Trans. Metall. Soc. AIME, 221, 1001 – 1008 (1961).
V. I. Chueshov, Industrial Technology of Drugs X [in Russian], MTK-Kniga, Izd. MFAU, Moscow (2002), p. 33.
PM of IKhFZ Co. LSR-003747 / 09-180509 “Ascorbic acid tablets, 0.1 g”.
I. M. Gracheva and Yu. P. Grachev, Laboratory Practicum for Enzyme Preparation Technology [in Russian], L and PP, Moscow (1982), pp. 214 – 217.
A. V. Filimonova, A. S. Gavrilov, and Yu. A. Tret’yakova, RU Pat. 2,567,508, Nov. 10, 2015; Byull. Izobret., No. 31 (2015).
Author information
Authors and Affiliations
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 52, No. 5, pp. 49 – 54, May, 2018.
Rights and permissions
About this article
Cite this article
Filimonova, A.V., Tret’yakova, Y.A. & Gavrilov, A.S. 3D-Modeling of the Influence of Isomalt, Glucose, and Compaction Pressure on the Quality of Ascorbic-Acid Tablets. Pharm Chem J 52, 467–472 (2018). https://doi.org/10.1007/s11094-018-1841-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-018-1841-2